Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus
- PMID: 9503337
- PMCID: PMC2230738
- DOI: 10.1111/j.1469-7793.1998.775bv.x
Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus
Abstract
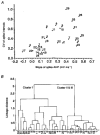
1. Whole cell patch clamp recordings of neurons in slices of the suprachiasmatic nucleus (SCN) were made in order to assess their electrophysiological and morphological heterogeneity. This assessment was accomplished by (i) quantification of intrinsic membrane properties recorded in current clamp mode, (ii) studying frequency distributions of these properties, (iii) grouping of cells based on visual inspection of data records, and (iv) use of cluster analysis methods. 2. Marked heterogeneity was found in the resting membrane potential, input resistance, time constant, rate of frequency adaptation, size of rebound depolarization (low-threshold Ca2+ potential) and regularity of firing. The frequency distribution of these membrane properties deviated significantly from a normal distribution. Other parameters, including spike amplitude and width, amplitude and rising slope of the spike after-hyperpolarization (AHP) and amplitude of the spike train AHP, showed considerable variability as well but generally obeyed a normal distribution. 3. Visual inspection of the data led to partitioning of cells into three clusters, viz. cluster I characterized by monophasic spike AHPs and irregular firing in the frequency range from 1.5 to 5.0 Hz; cluster II with biphasic spike AHPs and regular firing in the same range; and cluster III with large rebound depolarizations and biphasic spike AHPs. In a post hoc analysis, these clusters also appeared to differ in other membrane properties. This grouping was confirmed by hierarchical tree clustering and multidimensional scaling. 4. The light microscopic properties of recorded neurons were studied by biocytin labelling. Neurons had monopolar, bipolar or multipolar branching patterns and were often varicose. Axons sometimes originated from distal dendritic segments and usually branched into multiple collaterals. Many cells with extra-SCN projections also possessed intranuclear axon collaterals. We found no morphological differences between clusters except that cluster III neurons possessed more axon collaterals than cluster I or II cells. 5. These results suggest that SCN neurons are heterogeneous in some basic as well as active membrane properties and can be partitioned into at least three clusters. Cluster I and II cells fire spontaneously in a regular and irregular mode, respectively, and sustain prolonged spike trains. In contrast, cluster III cells have low firing rates but may adopt a burst-like firing mode when receiving appropriate input. While all clusters transmit output to target cells within and outside SCN, cluster III cells in particular are suggested to affect excitability of large numbers of SCN neurons by their extensive local network of axon collaterals.
Figures










References
-
- Akasu T, Shoji S, Hasuo H. Inward rectifier and low-threshold calcium currents contribute to the spontaneous firing mechanism in neurons of the rat suprachiasmatic nucleus. Pflügers Archiv. 1993;425:109–116. - PubMed
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. Journal of Neuroscience Methods. 1989;30:203–210. - PubMed
-
- Buijs RM, Wortel J, Hou Y-X. Colocalization of gamma-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. Journal of Comparative Neurology. 1995;358:343–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

