Altered regulation of natriuretic peptides in the rat heart by prenatal exposure to morphine
- PMID: 9503344
- PMCID: PMC2230745
- DOI: 10.1111/j.1469-7793.1998.867bv.x
Altered regulation of natriuretic peptides in the rat heart by prenatal exposure to morphine
Abstract
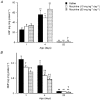
1. Both endogenous and exogenous opioids modulate blood pressure and cardiac function by stimulating cardiac synthesis of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP). Since morphine crosses the placental barrier, it could alter the ANF-BNP system in the fetal heart. The aim of this study was to characterize cardiac natriuretic peptides in normal rat development and in rats prenatally exposed to morphine. 2. Female rats received either saline or morphine (10 or 20 mg kg-1 day-1) via osmotic minipumps during gestation. The effects of this treatment were investigated in offspring at 1, 4 and 22 days of age. 3. During maturation, atrial ANF and ANF mRNA increased by 3-fold from birth to 3 weeks of age, but BNP and BNP mRNA tended to decrease. In the ventricles, both ANF and BNP content decreased at 3 weeks after birth, from 25.11 +/- 3.6 to 0.81 +/- 0.1 ng (mg protein)-1 (P < 0.001), and from 3.36 +/- 0.33 to 0.19 +/- 0.01 ng (mg protein)-1 (P < 0.001), respectively. However, whereas ventricular ANF mRNA decreased, BNP mRNA levels did not change during maturation. Prenatal exposure to morphine significantly increased ANF content in the left atria of 22-day-old rats, and in the right atria of 1-, 4- and 22-day-old rats compared with age-matched saline controls. In contrast, prenatal exposure to 20 mg kg-1 day-1 morphine significantly inhibited BNP and BNP mRNA in the ventricles at all ages studied. 4. These observations suggest that alterations in mRNA synthesis or stability and/or post-translational processing of ANF and BNP occur in the heart during maturation, and that prenatal exposure to morphine alters cardiac production, and possibly release, of both peptides.
Figures


References
-
- Aburaya M, Suzuki E, Minamino N, Kangawa K, Tanaka K, Matsuo H. Concentration and molecular forms of brain natriuretic peptide in rat plasma and spinal cord. Biochemical and Biophysical Research Communications. 1991;177:40–47. - PubMed
-
- Bloch KD, Seidman JG, Naftilan JD, Fallon JT, Seidman CE. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell. 1986;47:695–702. - PubMed
-
- Cameron VA, Aitken GD, Ellmers LJ, Kennedy MA, Espiner EA. The sites of gene expression of atrial, brain, and C-type natriuretic peptides in mouse fetal development: Temporal changes in embryos and placenta. Endocrinology. 1996;137:817–823. - PubMed
-
- Cheung BMY, Dickerson JEC, Ashby MJ, Brown MJ, Brown J. Effects of physiological increments in human α-atrial natriuretic peptide and human brain natriuretic peptide in normal male subjects. Clinical Science. 1994;86:723–730. - PubMed
-
- Collins H, DiCarlo S. Attenuation of postexertional hypotension by cardiac afferent blockade. American Journal of Physiology. 1993;265:H1179–1183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

