The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation
- PMID: 9508770
- PMCID: PMC2132678
- DOI: 10.1083/jcb.140.6.1369
The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation
Abstract
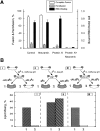
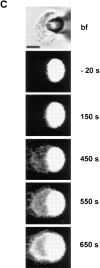
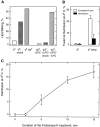
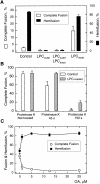
The mechanism of bilayer unification in biological fusion is unclear. We reversibly arrested hemagglutinin (HA)-mediated cell-cell fusion right before fusion pore opening. A low-pH conformation of HA was required to form this intermediate and to ensure fusion beyond it. We present evidence indicating that outer monolayers of the fusing membranes were merged and continuous in this intermediate, but HA restricted lipid mixing. Depending on the surface density of HA and the membrane lipid composition, this restricted hemifusion intermediate either transformed into a fusion pore or expanded into an unrestricted hemifusion, without pores but with unrestricted lipid mixing. Our results suggest that restriction of lipid flux by a ring of activated HA is necessary for successful fusion, during which a lipidic fusion pore develops in a local and transient hemifusion diaphragm.
Figures
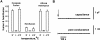
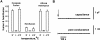
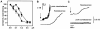



References
-
- Anderson CM, Georgiou GN, Morrison IE, Stevenson GV, Cherry RJ. Tracking of cell surface receptors by fluorescence digital imaging microscopy using a charge-coupled device camera. Low-density lipoprotein and influenza virus receptor mobility at 4°C. J Cell Sci. 1992;101:415–425. - PubMed
-
- Bentz J, Ellens H, Alford D. An architecture for the fusion site of influenza hemagglutinin. FEBS Lett. 1990;276:1–5. - PubMed
-
- Bierbaum TJ, Bouma SR, Huestis WH. A mechanism of erythrocyte lysis by lysophosphatidylcholine. Biochim Biophys Acta. 1979;555:102–110. - PubMed
-
- Blumenthal R, Pak CC, Raviv Y, Krumbiegel M, Bergelson LD, Morris SJ, Lowy RJ. Transient domains induced by influenza haemagglutinin during membrane fusion. Mol Membr Biol. 1995;12:135–142. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

