Processivity of the motor protein kinesin requires two heads
- PMID: 9508772
- PMCID: PMC2132675
- DOI: 10.1083/jcb.140.6.1395
Processivity of the motor protein kinesin requires two heads
Abstract
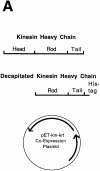
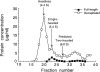
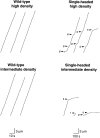
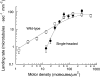
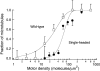
A single kinesin molecule can move for hundreds of steps along a microtubule without dissociating. One hypothesis to account for this processive movement is that the binding of kinesin's two heads is coordinated so that at least one head is always bound to the microtubule. To test this hypothesis, the motility of a full-length single-headed kinesin heterodimer was examined in the in vitro microtubule gliding assay. As the surface density of single-headed kinesin was lowered, there was a steep fall both in the rate at which microtubules landed and moved over the surface, and in the distance that microtubules moved, indicating that individual single-headed kinesin motors are not processive and that some four to six single-headed kinesin molecules are necessary and sufficient to move a microtubule continuously. At high ATP concentration, individual single-headed kinesin molecules detached from microtubules very slowly (at a rate less than one per second), 100-fold slower than the detachment during two-headed motility. This slow detachment directly supports a coordinated, hand-over-hand model in which the rapid detachment of one head in the dimer is contingent on the binding of the second head.
Figures
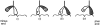


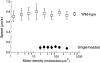


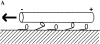
References
-
- Berliner E, Young EC, Anderson K, Mahtani HK, Gelles J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature. 1995;373:718–721. - PubMed
-
- Block SM, Goldstein LS, Schnapp BJ. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. - PubMed
-
- Bloom GS, Endow SA. Motor proteins 1: kinesins. Protein Profile. 1995;2:1105–1171. - PubMed
-
- Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–3416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

