Role of the kinesin neck region in processive microtubule-based motility
- PMID: 9508773
- PMCID: PMC2132664
- DOI: 10.1083/jcb.140.6.1407
Role of the kinesin neck region in processive microtubule-based motility
Abstract
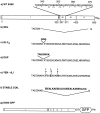
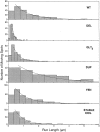
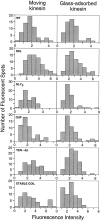
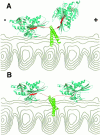
Kinesin is a dimeric motor protein that can move along a microtubule for several microns without releasing (termed processive movement). The two motor domains of the dimer are thought to move in a coordinated, hand-over-hand manner. A region adjacent to kinesin's motor catalytic domain (the neck) contains a coiled coil that is sufficient for motor dimerization and has been proposed to play an essential role in processive movement. Recent models have suggested that the neck enables head-to-head communication by creating a stiff connection between the two motor domains, but also may unwind during the mechanochemical cycle to allow movement to new tubulin binding sites. To test these ideas, we mutated the neck coiled coil in a 560-amino acid (aa) dimeric kinesin construct fused to green fluorescent protein (GFP), and then assayed processivity using a fluorescence microscope that can visualize single kinesin-GFP molecules moving along a microtubule. Our results show that replacing the kinesin neck coiled coil with a 28-aa residue peptide sequence that forms a highly stable coiled coil does not greatly reduce the processivity of the motor. This result argues against models in which extensive unwinding of the coiled coil is essential for movement. Furthermore, we show that deleting the neck coiled coil decreases processivity 10-fold, but surprisingly does not abolish it. We also demonstrate that processivity is increased by threefold when the neck helix is elongated by seven residues. These results indicate that structural features of the neck coiled coil, although not essential for processivity, can tune the efficiency of single molecule motility.
Figures
References
-
- Amos LA, Hirose K. The structure of microtubule-motor complexes. Curr Opin Cell Biol. 1997;9:4–11. - PubMed
-
- Arnal I, Metoz F, DeBonis S, Wade RH. Three-dimensional structure of functional motor proteins on microtubules. Curr Biol. 1996;6:1265–1270. - PubMed
-
- Berliner E, Young EC, Anderson K, Mahtani H, Gelles J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature. 1995;373:718–721. - PubMed
-
- Block SM. Fifty ways to love your lever: myosin motors. Cell. 1996;87:151–157. - PubMed
-
- Block SM, Goldstein LS, Schnapp BJ. Bead movement by single kinesin molecules with optical tweezers. Nature. 1990;348:348–352. - PubMed

