Dibasic protein kinase A sites regulate bursting rate and nucleotide sensitivity of the cystic fibrosis transmembrane conductance regulator chloride channel
- PMID: 9508802
- PMCID: PMC2230889
- DOI: 10.1111/j.1469-7793.1998.365bq.x
Dibasic protein kinase A sites regulate bursting rate and nucleotide sensitivity of the cystic fibrosis transmembrane conductance regulator chloride channel
Abstract
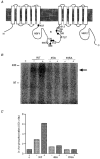
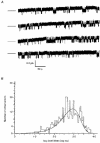
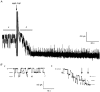
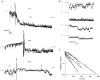
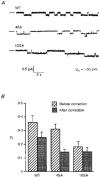
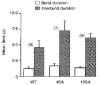
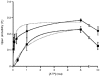
1. The relationship between phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel and its gating by nucleotides was examined using the patch clamp technique by comparing strongly phosphorylated wild-type (WT) channels with weakly phosphorylated mutant channels lacking four (4SA) or all ten (10SA) dibasic consensus sequences for phosphorylation by protein kinase A (PKA). 2. The open probability (Po) of strongly phosphorylated WT channels in excised patches was about twice that of 4SA and 10SA channels, after correcting for the number of functional channels per patch by addition of adenylylimidodiphosphate (AMP-PNP). The mean burst durations of WT and mutant channels were similar, and therefore the elevated Po of WT was due to its higher bursting rate. 3. The ATP dependence of the 10SA mutant was shifted to higher nucleotide concentrations compared with WT channels. The relationship between Po and [ATP] was noticeably sigmoid for 10SA channels (Hill coefficient, 1.8), consistent with positive co-operativity between two sites. Increasing ATP concentration to 10 mM caused the Po of both WT and 10SA channels to decline. 4. Wild-type and mutant CFTR channels became locked in open bursts when exposed to mixtures of ATP and the non-hydrolysable analogue AMP-PNP. The rate at which the low phosphorylation mutants became locked open was about half that of WT channels, consistent with Po being the principal determinant of locking rate in WT and mutant channels. 5. We conclude that phosphorylation at 'weak' PKA sites is sufficient to sustain the interactions between the ATP binding domains that mediate locking by AMP-PNP. Phosphorylation of the strong dibasic PKA sites controls the bursting rate and Po of WT channels by increasing the apparent affinity of CFTR for ATP.
Figures
References
-
- Anderson M P, Berger H A, Rich D P, Gregory R J, Smith A E, Welsh M J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. - PubMed
-
- Baukrowitz T, Hwang T-C, Nairn A C, Gadsby D C. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. - PubMed
-
- Berger H A, Travis S M, Welsh M J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and phosphatases. Journal of Biological Chemistry. 1993;268:2037–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

