Barium triggers rapid endocytosis in calf adrenal chromaffin cells
- PMID: 9508811
- PMCID: PMC2230899
- DOI: 10.1111/j.1469-7793.1998.483bq.x
Barium triggers rapid endocytosis in calf adrenal chromaffin cells
Abstract
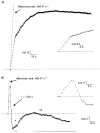
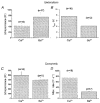
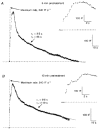
1. Changes in cell capacitance were monitored in whole-cell patch-clamp recordings from calf adrenal chromaffin cells using a software-based phase-tracking technique. Rapid endocytosis and exocytosis were observed in extracellular solutions containing either Ca2+ or Ba2+. 2. There was no significant difference in the magnitude or the time course of rapid endocytosis of cells stimulated in Ca2+ as compared to Ba2+. When cells were pretreated with caffeine and thapsigargin in order to deplete intracellular Ca2+ stores, rapid endocytosis in Ba2+ was not affected. This indicates that Ba2+ itself is capable of supporting rapid endocytosis. 3. The application of the calmodulin inhibitor calmidazolium via the intracellular pipette solution did not inhibit rapid endocytosis. Although our findings are inconsistent with an immediate requirement for calmodulin in rapid endocytosis, they do not rule out an involvement on a longer time scale. 4. While rapid endocytosis was not affected by the substitution of Ca2+ with Ba2+, the maximum rate of exocytosis was higher in cells stimulated in Ca2+ than in Ba2+. Since Ba2+ currents were much larger than Ca2+ currents during depolarizations to +10 mV (the test potential used in these experiments), Ba2+ appears to be less efficient at promoting exocytosis than Ca2+.
Figures



Similar articles
-
Effects of divalent cations on exocytosis and endocytosis from single mouse pancreatic beta-cells.J Physiol. 1995 Sep 1;487 ( Pt 2)(Pt 2):465-77. doi: 10.1113/jphysiol.1995.sp020893. J Physiol. 1995. PMID: 8558477 Free PMC article.
-
Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells.J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):591-608. doi: 10.1111/j.1469-7793.1998.591bv.x. J Physiol. 1998. PMID: 9503324 Free PMC article.
-
The mechanism of Ba(2+)-induced exocytosis from single chromaffin cells.FEBS Lett. 1993 Dec 20;336(1):48-52. doi: 10.1016/0014-5793(93)81606-z. FEBS Lett. 1993. PMID: 8262215
-
Control of exocytosis in adrenal chromaffin cells by GTP-binding proteins studied using permeabilized cells and patch-clamp capacitance measurements.Biochem Soc Trans. 1994 May;22(2):468-71. doi: 10.1042/bst0220468. Biochem Soc Trans. 1994. PMID: 7958348 Review. No abstract available.
-
Spatio-temporal regulation of neurotransmitter release by PKC; studies in adrenal chromaffin cells.Crit Rev Neurobiol. 2004;16(1-2):173-9. doi: 10.1615/critrevneurobiol.v16.i12.180. Crit Rev Neurobiol. 2004. PMID: 15581412 Review.
Cited by
-
Increases in intracellular pH facilitate endocytosis and decrease availability of voltage-gated proton channels in osteoclasts and microglia.J Physiol. 2013 Dec 1;591(23):5851-66. doi: 10.1113/jphysiol.2013.263558. Epub 2013 Sep 30. J Physiol. 2013. PMID: 24081153 Free PMC article.
-
Rapid endocytosis and vesicle recycling in neuroendocrine cells.Cell Mol Neurobiol. 2010 Nov;30(8):1365-70. doi: 10.1007/s10571-010-9579-8. Epub 2010 Nov 3. Cell Mol Neurobiol. 2010. PMID: 21046457 Free PMC article. Review.
-
Sustained stimulation of exocytosis triggers continuous membrane retrieval in rat pituitary somatotrophs.J Physiol. 2001 May 1;532(Pt 3):771-83. doi: 10.1111/j.1469-7793.2001.0771e.x. J Physiol. 2001. PMID: 11313445 Free PMC article.
-
Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal.Nat Neurosci. 2009 Aug;12(8):1003-1010. doi: 10.1038/nn.2355. Epub 2009 Jul 26. Nat Neurosci. 2009. PMID: 19633667 Free PMC article.
-
Regulation by L-type calcium channels of endocytosis: an overview.J Mol Neurosci. 2012 Oct;48(2):360-7. doi: 10.1007/s12031-012-9786-5. Epub 2012 May 12. J Mol Neurosci. 2012. PMID: 22581437 Review.
References
-
- Artalejo C R, Elhamdani A, Palfrey H C. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. 10.1016/S0896-6273(00)80036-7. - DOI - PubMed
-
- Artalejo C R, Perlman R L, Fox A P. Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the ‘classic’ N type. Neuron. 1992;8:85–95. 10.1016/0896-6273(92)90110-Y. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

