Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries
- PMID: 9508817
- PMCID: PMC2230883
- DOI: 10.1111/j.1469-7793.1998.561bq.x
Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries
Abstract
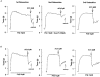
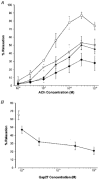
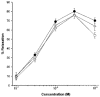
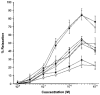
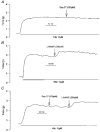
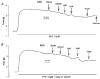
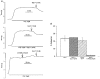
1. The contribution of gap junctions to endothelium-dependent relaxation was investigated in isolated rabbit conduit artery preparations pre-constricted by 10 microM phenylephrine (PhE). 2. Acetylcholine (ACh) relaxed the thoracic aorta by approximately 60 % and the superior mesenteric artery (SMA) by approximately 90 %. A peptide possessing sequence homology with extracellular loop 2 of connexin 43 (Gap 27, 300 microM) inhibited relaxation by approximately 40 % in both artery types. Gap 27 also attenuated the endothelium-dependent component of the relaxation induced by ATP in thoracic aorta but did not modify force development in response to PhE. 3. NG-nitro-L-arginine methyl ester (L-NAME, 300 microM), an inhibitor of NO synthase, attenuated ACh-induced relaxation by approximately 90 % in the aorta but only by approximately 40 % in SMA (P < 0.05). Residual L-NAME-insensitive relaxations were almost abolished by 300 microM Gap 27 in aorta and inhibited in a concentration-dependent fashion in SMA (approximately 50 % at 100 microM and approximately 80 % at 10 mM). Gap 27 similarly attenuated the endothelium-dependent component of L-NAME-insensitive relaxations to ATP in aorta. 4. Responses to cyclopiazonic acid, which stimulates endothelium-dependent relaxation through a receptor-independent mechanism, were also attenuated by Gap 27, whereas this peptide exerted no effect on the NO-mediated relaxation induced by sodium nitroprusside in preparations denuded of endothelium. 5. ACh-induced relaxation of 'sandwich' mounts of aorta or SMA were unaffected by Gap 27 but completely abolished by L-NAME. 6. We conclude that direct heterocellular communication between the endothelium and smooth muscle contributes to endothelium-dependent relaxations evoked by both receptor-dependent and -independent mechanisms. The inhibitory effects of Gap 27 peptide do not involve homocellular communication within the vessel wall or modulation of NO release or action.
Figures
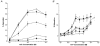
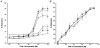
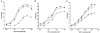
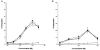
References
-
- Adeagbo A S, Triggle C R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. Journal of Cardiovascular Pharmacology. 1993;21:423–429. - PubMed
-
- Bény J-L, Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. American Journal of Physiology. 1994;266:H1465–1472. - PubMed
-
- Bolotina V M, Najibi S, Palacino J J, Pagano P J, Cohen R A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
-
- Brayden J E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. American Journal of Physiology. 1990;259:H668–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

