Focally recorded single sympathetic postganglionic neuronal activity supplying rat lateral tail vein
- PMID: 9508818
- PMCID: PMC2230877
- DOI: 10.1111/j.1469-7793.1998.575bq.x
Focally recorded single sympathetic postganglionic neuronal activity supplying rat lateral tail vein
Abstract
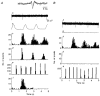
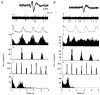
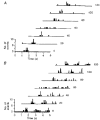
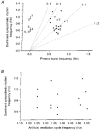
1. In anaesthetized rats, using a focal recording technique, activity was recorded from single sympathetic postganglionic neurones innervating the lateral tail veins. On-going activity was examined in order to determine whether it had similar or different characteristics to those recorded from the caudal ventral artery in a previous study. 2. Animals were artificially ventilated, vagotomized, paralysed and given a pneumothorax. 3. The discharges of fourteen out of seventeen sympathetic postganglionic neurones were rhythmic. Such units had a mean firing frequency of 1.62 +/- 0. 70 Hz. The mean frequency of the dominant sympathetic rhythm under control conditions was 0.82 +/- 0.05 Hz. 4. The frequency of the dominant sympathetic rhythm was different from that of the phrenic rhythm in nine out of fourteen cases. 5. The mean frequency of the dominant sympathetic rhythm was: (i) not influenced significantly by hypocapnic apnoea, (ii) decreased by hyperthermia, which increased the frequency of the phrenic rhythm, (iii) in all cases different from that of the artificial ventilation cycle. 6. The above characteristics are similar to those recorded from the sympathetic supply to the caudal ventral artery of the same vascular bed under comparable conditions.
Figures
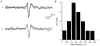


References
-
- Andrews T J. PhD Thesis. University of London; 1993. The role of targets and growth factors in neuronal ageing.
-
- Aukland K, Wiig H. Haemodynamics and interstitial fluid pressure in the rat tail. American Journal of Physiology. 1984;247:H80–87. - PubMed
-
- Bevan J A, Bevan R D, Duckles S P. Adrenergic regulation of smooth muscle. In: Shepherd J T, Abboud F M, editors. Handbook of Physiology, section 2, The Cardiovascular System. III. Bethesda, MD, USA: American Physiological Society; 1986. pp. 515–566. part 1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

