Functional expression of the Ile693Thr Na+ channel mutation associated with paramyotonia congenita in a human cell line
- PMID: 9508833
- PMCID: PMC2230833
- DOI: 10.1111/j.1469-7793.1998.721bs.x
Functional expression of the Ile693Thr Na+ channel mutation associated with paramyotonia congenita in a human cell line
Abstract
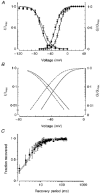
1. The Ile693Thr mutation of the skeletal muscle Na+ channel alpha-subunit is associated with an unusual phenotype of paramyotonia congenita characterized by cold-induced muscle weakness but no stiffness. This mutation occurs in the S4-S5 linker of domain II, a region that has not been previously implicated in paramyotonia congenita. 2. The Ile693Thr mutation was introduced into the human skeletal muscle Na+ gene for functional expression in human embryonic kidney (HEK) cells. The currents expressed were recorded with the whole-cell voltage-clamp technique. 3. In comparison with wild-type currents, Ile693Thr mutant currents showed a clear shift of about -9 mV in the voltage dependence of activation. 4. In contrast to other mutations of the Na+ channel known to cause paramyotonia congenita, the Ile693Thr mutation did not induce any significant change in the kinetics, nor in the voltage dependence, of fast inactivation. 5. In conclusion, this study provides further evidence of the involvement of the S4-S5 linker in the voltage dependence of Na+ channel activation. The negative shift in the voltage dependence found in this mutation must be associated to other defects, plausibly an impairment of the slow inactivation, to account for the long periods of muscle weakness experienced by the patients.
Figures


Similar articles
-
A1152D mutation of the Na+ channel causes paramyotonia congenita and emphasizes the role of DIII/S4-S5 linker in fast inactivation.J Physiol. 2005 Jun 1;565(Pt 2):415-27. doi: 10.1113/jphysiol.2004.081018. Epub 2005 Mar 24. J Physiol. 2005. PMID: 15790667 Free PMC article.
-
Mutant channels contribute <50% to Na+ current in paramyotonia congenita muscle.Brain. 1999 Jun;122 ( Pt 6):1085-92. doi: 10.1093/brain/122.6.1085. Brain. 1999. PMID: 10356061
-
Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans.J Physiol. 2004 Feb 1;554(Pt 3):635-47. doi: 10.1113/jphysiol.2003.053082. Epub 2003 Nov 14. J Physiol. 2004. PMID: 14617673 Free PMC article.
-
From mutation to myotonia in sodium channel disorders.Neuromuscul Disord. 1997 Jun;7(4):241-9. doi: 10.1016/s0960-8966(97)00430-6. Neuromuscul Disord. 1997. PMID: 9196906 Review.
-
[A family of paramyotonia congenita].Rinsho Shinkeigaku. 1993 Apr;33(4):452-4. Rinsho Shinkeigaku. 1993. PMID: 8396519 Review. Japanese.
Cited by
-
Up-regulation of voltage-gated sodium channels by peptides mimicking S4-S5 linkers reveals a variation of the ligand-receptor mechanism.Sci Rep. 2020 Apr 3;10(1):5852. doi: 10.1038/s41598-020-62615-6. Sci Rep. 2020. PMID: 32246066 Free PMC article.
-
Activation and inactivation of the voltage-gated sodium channel: role of segment S5 revealed by a novel hyperkalaemic periodic paralysis mutation.J Neurosci. 1999 Jun 15;19(12):4762-71. doi: 10.1523/JNEUROSCI.19-12-04762.1999. J Neurosci. 1999. PMID: 10366610 Free PMC article.
-
Mechanisms of a human skeletal myotonia produced by mutation in the C-terminus of NaV1.4: is Ca2+ regulation defective?PLoS One. 2013 Dec 6;8(12):e81063. doi: 10.1371/journal.pone.0081063. eCollection 2013. PLoS One. 2013. PMID: 24324661 Free PMC article.
-
Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy.J Neurosci. 2004 Sep 22;24(38):8232-6. doi: 10.1523/JNEUROSCI.2695-04.2004. J Neurosci. 2004. PMID: 15385606 Free PMC article.
-
Divalent cation-responsive myotonia and muscle paralysis in skeletal muscle sodium channelopathy.Neuromuscul Disord. 2015 Nov;25(11):908-12. doi: 10.1016/j.nmd.2015.08.007. Epub 2015 Aug 20. Neuromuscul Disord. 2015. PMID: 26494408 Free PMC article.
References
-
- Armstrong CM, Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the sodium channels. Journal of General Physiology. 1974;63:533–553. 10.1085/jgp.63.5.533. - DOI - PMC - PubMed
-
- Chahine M, George AL, Jr, Zhou M, Ji S, Sun W, Barchi RL, Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994;12:281–294. - PubMed
-
- Cummins TR, Zhou J, Sigworth FJ, Ukomadu C, Stephan M, Ptacek LJ, Agnew WS. Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron. 1993;10:667–678. - PubMed
-
- Feero WG, Wang J, Barany F, Zhou J, Todorovic SM, Convit R, Galloway G, Hartlage P, Hayakawa H, Hoffman EP. Hyperkalemic periodic paralysis: rapid molecular diagnosis and relationship of genotype to phenotype in 12 families. Neurology. 1993;43:668–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

