The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes
- PMID: 9508837
- PMCID: PMC2230826
- DOI: 10.1111/j.1469-7793.1998.759bs.x
The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes
Abstract
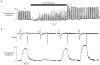
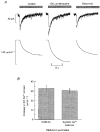
1. The effects of tetracaine were examined on rat ventricular myocytes. In both field-stimulated and voltage-clamped cells tetracaine (100-200 microM) produced an initial decrease of contraction before a recovery towards the control level. Removal of tetracaine produced a transient overshoot of contraction to levels greater than the control. 2. The transient decrease of contraction produced by tetracaine was accompanied by a small transient increase in the integral of the L-type Ca2+ current and a larger transient decrease of the Na+-Ca2+ exchange current on repolarization. These are attributed to decreased systolic release of Ca2+. On removal of tetracaine there was an increase of the Na+-Ca2+ exchange current. Before the addition of tetracaine, calculated Ca2+ influx and efflux across the sarcolemma were approximately equal. On adding tetracaine, efflux was transiently less than influx and, on removal of tetracaine, efflux was greater than influx. 3. These changes in Ca2+ fluxes result in an increase of cell Ca2+ during exposure to tetracaine. The calculated magnitude of this increase was equal to that measured directly by applying caffeine (20 mM) to release sarcoplasmic reticulum (SR) Ca2+ and integrating the resulting Na+-Ca2+ exchange current. 4. It is concluded that the effects of tetracaine can be accounted for by depression of calcium-induced Ca2+ release (CICR). The response is transient because the inhibition is compensated for by an increase of SR Ca2+ content such that there is no steady-state effect on the magnitude of the systolic Ca2+ transient. The consequences of this result for the effects of other modulators of CICR are discussed.
Figures

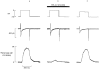



References
-
- Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. Journal of General Physiology. 1996;108:435–454. 10.1085/jgp.108.5.435. - DOI - PMC - PubMed
-
- Bassani JWM, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. American Journal of Physiology. 1995;268:C1313–1319. - PubMed
-
- Bridge JH, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science. 1990;248:376–378. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

