Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones
- PMID: 9508839
- PMCID: PMC2230818
- DOI: 10.1111/j.1469-7793.1998.783bs.x
Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones
Abstract
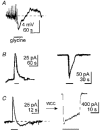
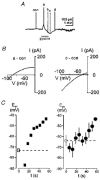
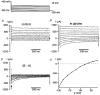
1. During early ontogeny, glycine receptors (GlyRs) exert depolarizing responses which may be of developmental relevance. We have used the gramicidin-perforated patch technique to elucidate the mechanism of glycine-activated currents in developing neurones of the rat lateral superior olive (LSO). 2. When the holding potential was set to -60 mV, perforated-patch recordings revealed glycine-induced inward currents in 59 %, outward currents in 5 % and biphasic currents in 34 % of the LSO neurones tested (n = 44). The biphasic currents were characterized by a transient outward phase which was followed by an inward phase. 3. Ion substitution experiments showed that both Cl- and HCO3- contributed to the glycine- induced biphasic current responses. 4. In the biphasic responses, the reversal potential of the glycine-induced current (Egly) depended on the response phase. A strong shift of Egly from a mean of -72 mV during the outward phase of the glycine response to a mean of -51 mV during the inward phase was observed, suggesting a shift of an ion gradient. 5. When the membrane potential was depolarized, 'tail' currents were induced in the presence of glycine. An increased duration or amplitude of the evoked depolarizations resulted in a proportional enlargement of these tail currents, indicating that they were produced by a shift of an ion gradient. Since changes of the HCO3- gradient are negligible, because of the carbonic anhydrase activity, we suggest that these tail currents were caused by a shift of the Cl- gradient. 6. We conclude that Cl- accumulates intracellularly during the activation of GlyRs and, consequently, Egly moves towards more positive values. 7. Coincident depolarizing stimuli enhanced intracellular Cl- accumulation and the shift of Egly, thereby switching hyperpolarizing to depolarizing action. This change could assist in an activity-dependent strengthening and refinement of glycinergic synapses during the maturation of inhibitory connectivity.
Figures


References
-
- Backus KH, Friauf E. Effects of synchronous depolarization on glycine-induced currents in developing rat auditory brainstem neurons. Society for Neuroscience Abstracts. 1996;22:647.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

