Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat
- PMID: 9508843
- PMCID: PMC2230817
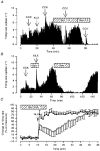
- DOI: 10.1111/j.1469-7793.1998.831bs.x
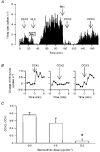
Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat
Abstract
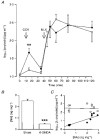
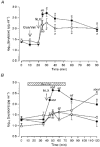
1. We have tested the hypothesis that morphine withdrawal excitation of oxytocin neurones that follows from administration of naloxone to morphine-dependent rats is a consequence of excitation of noradrenergic neurones. 2. Female rats were made morphine dependent by intracerebroventricular (i.c.v.) infusion of the opioid at increasing doses over 5 days. On the sixth day, the rats were anaesthetized with urethane or pentobarbitone and prepared for blood sampling to determine plasma oxytocin by radioimmunoassay or for in vivo extracellular recording of the firing rate of identified oxytocin neurones from the supraoptic nucleus. Morphine withdrawal was induced by intravenous (i.v.) injection of the opioid antagonist naloxone (5 mg kg-1). 3. In one group of rats the noradrenergic projections to the hypothalamus were lesioned by i.c.v. injection of 6-hydroxydopamine immediately prior to the induction of morphine dependence. In these rats the oxytocin secretion induced by i.v. cholecystokinin was reduced to 9 % of that seen in sham-lesioned rats but in contrast, no attenuation of morphine withdrawal-induced oxytocin secretion was observed. 4. i.c.v. infusion of the alpha1-adrenoreceptor antagonist benoxathian, at up to 5.3 microg min-1, dose- dependently inhibited the withdrawal excitation of oxytocin neurones in morphine-dependent rats under urethane anaesthesia, and benoxathian reduced withdrawal-induced oxytocin secretion to 37 % of that of vehicle-infused rats. i.c.v. benoxathian also inhibited the activity of oxytocin neurones in morphine-naïve rats. Similarly, microdialysis administration of 2 mM benoxathian directly onto the surface of the supraoptic nucleus reduced the activity of oxytocin neurones by 53 %. 5. Thus noradrenergic systems are not essential for the expression of morphine withdrawal excitation, since chronic neurotoxic destruction of the noradrenergic inputs to the hypothalamus did not affect the magnitude of withdrawal-induced oxytocin secretion. However, tonically active noradrenergic inputs influence the excitability of oxytocin neurones, and acute antagonism of this noradrenergic tone can powerfully impair the ability of oxytocin neurones to exhibit morphine withdrawal excitation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

