Activity-dependent hyperpolarization of human motor axons produced by natural activity
- PMID: 9508850
- PMCID: PMC2230820
- DOI: 10.1111/j.1469-7793.1998.919bs.x
Activity-dependent hyperpolarization of human motor axons produced by natural activity
Abstract
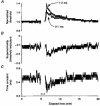
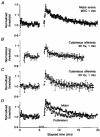
1. The changes in excitability of motor axons produced by natural activity were measured in six healthy subjects using voluntary contractions lasting 15 s, 30 s and 1 min, by recording the changes in stimulus current required to produce a compound muscle action potential of approximately 60 % of maximum. 2. On cessation of the contractions there was a prominent increase in the current required to produce the target potential, accompanied by an increase in rheobase, a decrease in strength-duration time constant, and an increase in axonal supernormality. These changes indicate that the hypoexcitability was due to axonal hyperpolarization. 3. The activity-dependent hypoexcitability increased in depth and duration the longer the contraction. Following a 1 min contraction, it produced a 24 % increase in threshold, waning over 15 min. The hypoexcitability was greater than in cutaneous afferents tetanized to produce an equivalent rate-dependent stress. 4. It is concluded that natural activity results in substantial hyperpolarization of active axons and that, for similar discharge rates, the degree of hyperpolarization is greater in motor axons than cutaneous afferents. The greater effect of activity on the excitability of motor axons could be due to less inward rectification and less persistent Na+ conductance than in sensory axons. It is suggested that motor axons may therefore be more susceptible than cutaneous afferents to conduction block at sites of impaired safety margin for impulse conduction.
Figures


References
-
- Applegate C, Burke D. Changes in excitability of human cutaneous afferents following prolonged high-frequency stimulation. Brain. 1989;112:147–164. - PubMed
-
- Auer RN, Bell RB, Lee MA. Neuropathy with onion bulb formations and pure motor manifestations. Canadian Journal of Neurological Sciences. 1989;16:194–197. - PubMed
-
- Baker MD, Bostock H. Low-threshold persistent sodium current in rat large dorsal root ganglion neurons in culture. Journal of Neurophysiology. 1997;77:1503–1513. - PubMed
-
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. Journal of Neurophysiology. 1983;50:1380–1392. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

