A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition
- PMID: 9512519
- PMCID: PMC316625
- DOI: 10.1101/gad.12.6.858
A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition
Abstract
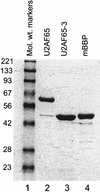
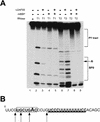
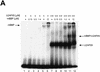
During the early events of pre-mRNA splicing, intronic cis-acting sequences are recognized and interact through a network of RNA-RNA, RNA-protein, and protein-protein contacts. Recently, we identified a branchpoint sequence binding protein in yeast (BBP). The mammalian ortholog (mBBP/SF1) also binds specifically to branchpoint sequences and interacts with the well studied mammalian splicing factor U2AF65, which binds to the adjacent polypyrimidine (PY) tract. In this paper we demonstrate that the mBBP/SF1-U2AF65 interaction promotes cooperative binding to a branchpoint sequence-polypyrimidine tract-containing RNA, and we suggest that this cooperative RNA binding contributes to initial recognition of the branchpoint sequence (BPS) during pre-mRNA splicing. We also demonstrate the essential nature of the third RBD of U2AF65 for the interaction between the two proteins, both in the presence and absence of RNA.
Figures













References
-
- Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. - PubMed
-
- Abovich N, Liao XC, Rosbash M. The yeast MUD2 protein: An interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes & Dev. 1994;8:843–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
