Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores
- PMID: 9515903
- PMCID: PMC107033
- DOI: 10.1128/JB.180.6.1375-1380.1998
Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores
Abstract
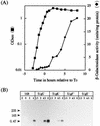
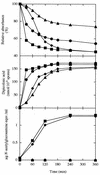
The predicted amino acid sequence of Bacillus subtilis ycbQ (renamed cwlJ) exhibits high similarity to those of the deduced C-terminal catalytic domain of SleBs, the specific cortex-hydrolyzing enzyme of B. cereus and the deduced one of B. subtilis. We constructed a cwlJ::lacZ fusion in the B. subtilis chromosome. The beta-galactosidase activity and results of Northern hybridization and primer extension analyses of the cwlJ gene indicated that it is transcribed by EsigmaE RNA polymerase. cwlJ-deficient spores responded to both L-alanine and AGFK, the A580 values of spore suspensions decreased more slowly than in the case of the wild-type strain, and the mutant spores released less dipicolinic acid than did those of the wild-type strain during germination. However, the mutant spores released only slightly less hexosamine than did the wild-type spores. In contrast, B. subtilis sleB spores did not release hexosamine at a significant level. While cwlJ and sleB spores were able to germinate, CJSB (cwlJ sleB) spores could not germinate but exhibited initial germination reactions, e.g., partial decrease in A580 and slow release of dipicolinic acid. CJSB spores became slightly gray after 6 h in the germinant, but their refractility was much greater than that of sleB mutant spores. The roles of the sleB and cwlJ mutations in germination and spore maturation are also discussed.
Figures

Similar articles
-
Activity and regulation of various forms of CwlJ, SleB, and YpeB proteins in degrading cortex peptidoglycan of spores of Bacillus species in vitro and during spore germination.J Bacteriol. 2013 Jun;195(11):2530-40. doi: 10.1128/JB.00259-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543708 Free PMC article.
-
Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex.J Appl Microbiol. 2009 Jul;107(1):318-28. doi: 10.1111/j.1365-2672.2009.04210.x. Epub 2009 Mar 10. J Appl Microbiol. 2009. PMID: 19302310
-
Proteins YlaJ and YhcN contribute to the efficiency of spore germination in Bacillus subtilis.FEMS Microbiol Lett. 2017 Apr 1;364(7). doi: 10.1093/femsle/fnx047. FEMS Microbiol Lett. 2017. PMID: 28333204
-
Spore Germination.Microbiol Spectr. 2015 Dec;3(6). doi: 10.1128/microbiolspec.TBS-0014-2012. Microbiol Spectr. 2015. PMID: 27337279 Review.
-
How do spores germinate?J Appl Microbiol. 2006 Sep;101(3):526-30. doi: 10.1111/j.1365-2672.2006.02885.x. J Appl Microbiol. 2006. PMID: 16907803 Review.
Cited by
-
Activity and regulation of various forms of CwlJ, SleB, and YpeB proteins in degrading cortex peptidoglycan of spores of Bacillus species in vitro and during spore germination.J Bacteriol. 2013 Jun;195(11):2530-40. doi: 10.1128/JB.00259-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543708 Free PMC article.
-
Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate.J Bacteriol. 2003 Apr;185(7):2315-29. doi: 10.1128/JB.185.7.2315-2329.2003. J Bacteriol. 2003. PMID: 12644503 Free PMC article.
-
The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis.J Bacteriol. 2002 Jan;184(2):584-7. doi: 10.1128/JB.184.2.584-587.2002. J Bacteriol. 2002. PMID: 11751839 Free PMC article.
-
Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores.J Bacteriol. 2001 Jul;183(13):3982-90. doi: 10.1128/JB.183.13.3982-3990.2001. J Bacteriol. 2001. PMID: 11395462 Free PMC article.
-
Roles of germination-specific lytic enzymes CwlJ and SleB in Bacillus anthracis.J Bacteriol. 2009 Apr;191(7):2237-47. doi: 10.1128/JB.01598-08. Epub 2009 Jan 30. J Bacteriol. 2009. PMID: 19181808 Free PMC article.
References
-
- Canosi U, Morelli G, Trautner T A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978;166:259–267. - PubMed
-
- Foster S J. Abstracts of the 9th International Conference on Bacilli. Lausanne, Switzerland: Universite de Lausanne; 1997. The role of cortex hydrolysis during spore germination; p. 42.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

