Frequency and cost of serious adverse drug reactions in a department of general medicine
- PMID: 9517375
- PMCID: PMC1873369
- DOI: 10.1046/j.1365-2125.1998.00667.x
Frequency and cost of serious adverse drug reactions in a department of general medicine
Abstract
Aims: To assess the frequency and cost of drug reactions causing or prolonging hospitalization.
Methods: All patients admitted to an internal medicine ward over 6 months were evaluated to identify serious adverse reactions. The number of drug classes on admission or at the time of the adverse drug reaction (ADR) was counted. Excess ADR-related hospital stay was computed using a) raw excess duration of hospital stay, b) correction of duration of hospital stay by age, sex, and number of drug classes, and c) estimation by investigator of excess hospital stay.
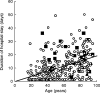
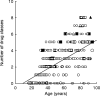
Results: Three hundred and twenty-nine patients were evaluated: 212 male, 117 female, mean age 57.2 (males: 52.2, females: 66.2 (P < 0.05)), range 17-95 years. They stayed a total of 3720 hospital days (mean stay 11.3 days). 298 had no ADR (mean age 55.8, taking a mean of 2.7 drug classes, 10.7 days hospital stay); 31 had ADRs: in 10, the ADR caused admission in patients with a mean age of 84 (P < 0.01 vs the two other groups), taking 6.3 drug classes, who stayed a mean of 15.1 days; 21 occurred in hospital in patients with a mean age of 63.6, taking 4.2 drug classes (P < 0.01), who stayed a mean of 19.2 days (P < 0.01 vs patients without ADRs). In four the ADR was fatal (13% of ADRs, 40% of deaths). Raw ADR-related excess hospital stay was 318 days (8.6% of all hospital days), after multivariate correction 282 days (7.6% of all hospital days), and with investigator estimation 197 days (5.3% of all hospital days). Point prevalence of ADRs at admission was 3%, incidence rate in hospital was 5.6/1000 patient-days.
Conclusions: 3% of the admissions were related to ADRs. In addition, 6.6% of hospitalized patients had significant ADRs. Between 5 and 9% of hospital costs were related to ADRs. In 24 of the 31 patients with ADRs (77%), these were related to the pharmacological properties of the involved drugs, and may possibly have been avoidable.
Figures
References
-
- Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–840. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266:2847–2851. - PubMed
-
- Harb GE, Alldredge BK, Coleman R, Jacobson MA. Pharmacoepidemiology of adverse drug reactions in hospitalized patients with human immunodeficiency virus disease. J Acquir Immune Defic Syndr. 1993;6:919–926. - PubMed
-
- Moore N, Briffaut C, Noblet C, Augustin-Normand C, Thuillez C. Indirect drug related costs. Lancet. 1995;345:588–589. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

