Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs
- PMID: 9517952
- PMCID: PMC105518
- DOI: 10.1128/AAC.42.3.687
Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs
Abstract
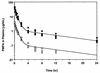
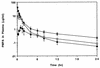
The pharmacokinetics, bioavailability, and metabolism of the anti-human immunodeficiency virus nucleotide analog 9[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) were determined in beagle dogs following intravenous, intraperitoneal, and oral administration. Fasted male beagle dogs (n = 5) were pretreated with pentagastrin and received PMPA (10 mg/kg of body weight) by the intravenous and oral routes with a washout period of 1 week between doses. A further group of male dogs received PMPA as a single dose via the intravenous (1 mg/kg; n = 5) and the intraperitoneal (10 mg/kg; n = 3) routes, with 1-week washout period between doses. The concentrations of PMPA in plasma and urine were determined over 48 h postdosing by fluorescence derivatization and high-performance liquid chromatography (HPLC). The potential for metabolism or biliary excretion of PMPA was evaluated in a dog with a chronic indwelling bile cannula. Urine, feces, and bile were collected at intervals over 48 h following the intravenous administration of [14C]PMPA (10 mg/kg; 55 microCi/kg). The concentrations of PMPA in plasma after intravenous injection were best described by an open two-compartment model with a terminal half-life of approximately 10 h. PMPA was excreted unchanged in urine (70%); recovery in feces (0.42%) or bile (0.26%) was negligible. The plasma clearance of PMPA (0.28+/-0.05 liter/h/kg) was substantially greater than the glomerular filtration rate in this species, suggesting active tubular secretion of PMPA. No metabolites of [14C]PMPA were observed in urine, feces, or bile on the basis of HPLC with radioactive flow detection. The remainder of the dose was probably excreted unchanged in urine beyond 48 h postdosing. The mean+/-standard deviation observed bioavailabilities of PMPA following oral and intraperitoneal administration at 10 mg/kg were 17.1%+/-1.88% and 73.5%+/-10.5%, respectively.
Figures

References
-
- Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antivir Res. 1993;8:261–272. - PMC - PubMed
-
- Barditch-Crovo P, Deeks S, Kahn J, Redpath I, Smith A, Hwang F, Hellmann N, Cundy K C, Rooney J, Lietman P S. Tenth International Conference on Antiviral Research. Latebreaker. 1997. PMPA: safety, pharmacokinetics, and antiretroviral activity when administered as a single dose and for seven consecutive days to patients with HIV infection.
-
- Bischofberger N, Naesens L, De Clercq E, Fridland A, Srinivas R V, Robbins B L, Arimilli M, Cundy K C, Kim C, Lacy S, Lee W A, Shaw J-P. Abstracts of the Fourth Conference on Retroviruses and Opportunistic Infections. Alexandria, Va: IDSA Foundation for Retrovirology and Human Health; 1997. Bis(POC)PMPA, an orally bioavailable prodrug of the antiretroviral agent PMPA, abstr. 214.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

