High-resolution restriction maps of bacterial artificial chromosomes constructed by optical mapping
- PMID: 9520376
- PMCID: PMC19846
- DOI: 10.1073/pnas.95.7.3390
High-resolution restriction maps of bacterial artificial chromosomes constructed by optical mapping
Abstract
Large insert clone libraries have been the primary resource used for the physical mapping of the human genome. Research directions in the genome community now are shifting direction from purely mapping to large-scale sequencing, which in turn, require new standards to be met by physical maps and large insert libraries. Bacterial artificial chromosome libraries offer enormous potential as the chosen substrate for both mapping and sequencing studies. Physical mapping, however, has come under some scrutiny as being "redundant" in the age of large-scale automated sequencing. We report the development and applications of nonelectrophoretic, optical approaches for high-resolution mapping of bacterial artificial chromosome that offer the potential to complement and thereby advance large-scale sequencing projects.
Figures
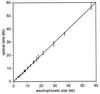


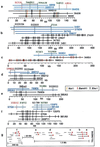


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

