Thiolate ligands in metallothionein confer redox activity on zinc clusters
- PMID: 9520391
- PMCID: PMC19861
- DOI: 10.1073/pnas.95.7.3478
Thiolate ligands in metallothionein confer redox activity on zinc clusters
Abstract
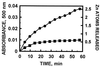
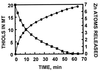
We postulate a novel and general mechanism in which the redox-active sulfur donor group of cyst(e)ine confers oxidoreductive characteristics on stable zinc sites in proteins. Thus, the present, an earlier, and accompanying manuscripts [Maret, W., Larsen, K. S. & Vallee, B. L. (1997) Proc. Natl. Acad. Sci. USA 94, 2233-2237; Jiang, L.-J., Maret, W. & Vallee, B. L. (1998) Proc. Natl. Acad. Sci. USA 95, 3483-3488; and Jacob, C., Maret, W. & Vallee, B. L. (1998) Proc. Natl. Acad. Sci. USA 95, 3489-3494] demonstrate that the interactive network featuring multiple zinc/sulfur bonds as found in the clusters of metallothionein (MT) constitutes a coordination unit critical for the concurrent oxidation of cysteine ligands and the ensuing release of zinc. The low position of MT (<-366 mV) on a scale of redox reagents allows its effective oxidation by relatively mild cellular oxidants, in particular disulfides. When MT is exposed to an excess of dithiodipyridine, all of its 20 cysteines are oxidized within 1 hr with the concomitant release of all 7 zinc atoms; similarly, the thiol/disulfide oxidoreductase DsbA reacts stoichiometrically with MT to release zinc. Zinc and sulfur ligands in the clusters are in a spatial arrangement that seemingly favors disulfide bond formation. Jointly, this and the above-mentioned manuscripts conclude that the control of cellular zinc distribution as a function of the energy state of the cell is the long sought role of MT. This specific MT function renders dubious the widely held belief that MT primarily scavenges radicals or detoxifies metals and is consistent with the frequent use of cysteine as a zinc ligand in proteins as a means of both tight and weak zinc binding of thiols and disulfides, respectively. Thus, we relate changes in the reducing power of the cell to the stability of the zinc/sulfur network in MT and the relative mobility of zinc and its control.
Figures


Comment in
-
Crosstalk of the group IIa and IIb metals calcium and zinc in cellular signaling.Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12325-7. doi: 10.1073/pnas.231481398. Proc Natl Acad Sci U S A. 2001. PMID: 11675482 Free PMC article. No abstract available.
References
-
- Robbins A H, McRee D E, Williamson M, Collett S A, Xuong N H, Furey W F, Wang B C, Stout C D. J Mol Biol. 1991;221:1269–1293. - PubMed
-
- Arseniev A, Schultze P, Wörgötter E, Braun W, Wagner G, Vašák M, Kägi J H R, Wüthrich K. J Mol Biol. 1988;201:637–657. - PubMed
-
- Vallee B L. Experientia Suppl. 1979;34:19–40. - PubMed
-
- Vallee B L. Experientia Suppl. 1987;52:5–16. - PubMed
-
- Vallee B L, Maret W. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 1–27.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

