23S rRNA positions essential for tRNA binding in ribosomal functional sites
- PMID: 9520399
- PMCID: PMC19869
- DOI: 10.1073/pnas.95.7.3525
23S rRNA positions essential for tRNA binding in ribosomal functional sites
Abstract
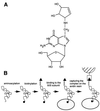
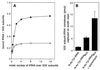
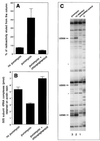
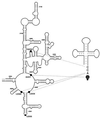
rRNA plays an important role in function of peptidyl transferase, the catalytic center of the ribosome responsible for the peptide bond formation. Proper placement of the peptidyl transferase substrates, peptidyl-tRNA and aminoacyl-tRNA, is essential for catalysis of the transpeptidation reaction and protein synthesis. In this report, we define a small set of rRNA nucleotides that are most likely directly involved in binding of tRNA in the functional sites of the large ribosomal subunit. By binding biotinylated tRNA substrates to randomly modified large ribosomal subunits from Escherichia coli and capturing resulting complexes on the avidin resin, we identified four nucleotides in the large ribosomal subunit rRNA (positions G2252, A2451, U2506, and U2585) whose modifications prevent binding of a peptidyl-tRNA analog in the P site and one residue (U2555) whose modification interferes with transfer of peptidyl moiety to puromycin. These nucleotides represent a subset of positions protected by tRNA analogs from chemical modification and significantly narrow the number of 23S rRNA nucleotides that may be directly involved in tRNA binding in the ribosomal functional sites.
Figures

References
-
- Moazed D, Noller H F. Cell. 1989;57:585–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

