Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL
- PMID: 9520411
- PMCID: PMC19881
- DOI: 10.1073/pnas.95.7.3597
Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL
Abstract
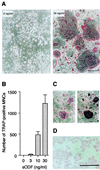
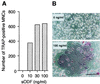
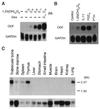
Osteoclasts, the multinucleated cells that resorb bone, develop from hematopoietic cells of monocyte/macrophage lineage. Osteoclast-like cells (OCLs) are formed by coculturing spleen cells with osteoblasts or bone marrow stromal cells in the presence of bone-resorbing factors. The cell-to-cell interaction between osteoblasts/stromal cells and osteoclast progenitors is essential for OCL formation. Recently, we purified and molecularly cloned osteoclastogenesis-inhibitory factor (OCIF), which was identical to osteoprotegerin (OPG). OPG/OCIF is a secreted member of the tumor necrosis factor receptor family and inhibits osteoclastogenesis by interrupting the cell-to-cell interaction. Here we report the expression cloning of a ligand for OPG/OCIF from a complementary DNA library of mouse stromal cells. The protein was found to be a member of the membrane-associated tumor necrosis factor ligand family and induced OCL formation from osteoclast progenitors. A genetically engineered soluble form containing the extracellular domain of the protein induced OCL formation from spleen cells in the absence of osteoblasts/stromal cells. OPG/OCIF abolished the OCL formation induced by the protein. Expression of its gene in osteoblasts/stromal cells was up-regulated by bone-resorbing factors. We conclude that the membrane-bound protein is osteoclast differentiation factor (ODF), a long-sought ligand mediating an essential signal to osteoclast progenitors for their differentiation into osteoclasts. ODF was found to be identical to TRANCE/RANKL, which enhances T-cell growth and dendritic-cell function. ODF seems to be an important regulator in not only osteoclastogenesis but also immune system.
Figures


Similar articles
-
A novel molecular mechanism modulating osteoclast differentiation and function.Bone. 1999 Jul;25(1):109-13. doi: 10.1016/s8756-3282(99)00121-0. Bone. 1999. PMID: 10423033
-
A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function.Biochem Biophys Res Commun. 1999 Mar 24;256(3):449-55. doi: 10.1006/bbrc.1999.0252. Biochem Biophys Res Commun. 1999. PMID: 10080918 Review.
-
Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand.Bone. 1999 Nov;25(5):517-23. doi: 10.1016/s8756-3282(99)00210-0. Bone. 1999. PMID: 10574571
-
Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures.Biochem Biophys Res Commun. 1998 May 8;246(1):199-204. doi: 10.1006/bbrc.1998.8586. Biochem Biophys Res Commun. 1998. PMID: 9600092
-
The molecular basis of osteoclast differentiation and activation.Novartis Found Symp. 2001;232:235-47; discussion 247-50. doi: 10.1002/0470846658.ch16. Novartis Found Symp. 2001. PMID: 11277084 Review.
Cited by
-
When the Damage Is Done: Injury and Repair in Thymus Function.Front Immunol. 2020 Aug 12;11:1745. doi: 10.3389/fimmu.2020.01745. eCollection 2020. Front Immunol. 2020. PMID: 32903477 Free PMC article. Review.
-
Mechanical strain-mediated reduction in RANKL expression is associated with RUNX2 and BRD2.Gene X. 2020 Jan 16;5:100027. doi: 10.1016/j.gene.2020.100027. eCollection 2020 Dec. Gene X. 2020. PMID: 32550554 Free PMC article.
-
Effects of denosumab on bone density, mass and strength in women with postmenopausal osteoporosis.Ther Adv Musculoskelet Dis. 2015 Jun;7(3):88-102. doi: 10.1177/1759720X15579189. Ther Adv Musculoskelet Dis. 2015. PMID: 26029270 Free PMC article. Review.
-
The regulation of bone turnover in ameloblastoma using an organotypic in vitro co-culture model.J Tissue Eng. 2016 Sep 29;7:2041731416669629. doi: 10.1177/2041731416669629. eCollection 2016 Jan-Dec. J Tissue Eng. 2016. PMID: 27746893 Free PMC article.
-
Deletion of Coagulation Factor IX Compromises Bone Mass and Strength: Murine Model of Hemophilia B (Christmas Disease).Calcif Tissue Int. 2021 Nov;109(5):577-585. doi: 10.1007/s00223-021-00872-x. Epub 2021 Jun 12. Calcif Tissue Int. 2021. PMID: 34117910 Free PMC article.
References
-
- Suda T, Takahashi N, Martin T J. Endocr Rev. 1992;13:66–80. - PubMed
-
- Suda T, Takahashi N, Martin T J. In: Endocrine Review Monographs. Bikle D D, Negrovilar A, editors. Vol. 4. Bethesda: Endocrine Soc.; 1995. pp. 266–270.
-
- Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Bone. 1995;17:87S–91S. - PubMed
-
- Roodman G D. Endocr Rev. 1996;17:308–332. - PubMed
-
- Takahashi N, Akatsu T, Uadagawa N, Sasaki T, Yamaguchi A, Moseley J M, Martin T J, Suda T. Endocrinology. 1988;123:2600–2602. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

