Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells
- PMID: 9520414
- PMCID: PMC19884
- DOI: 10.1073/pnas.95.7.3615
Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells
Abstract
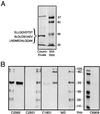
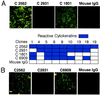
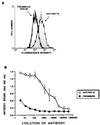
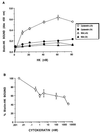
A kininogen binding protein(s), a putative receptor, was identified on endothelial cells. A 54-kDa protein was isolated by a biotin-high molecular mass kininogen (HK) affinity column that, on aminoterminal sequencing of tryptic digests, was identified as cytokeratin 1. Multiple antibodies directed to cytokeratin 1 reacted with a 54-kDa band on immunoblot of lysates of endothelial cells. On laser scanning confocal microscopy, cytokeratin 1 antigen was found on the surface of endothelial cells. Cytokeratin 1 antigen also was detected on endothelial cell membranes by flow cytometry. Moreover, an antipeptide antibody to a sequence unique to cytokeratin 1 also specifically bound to nonpermeabilized endothelial cells. Biotin-HK specifically bound to cytokeratin only in the presence of Zn2+, and cytokeratin blocked biotin-HK binding to endothelial cells. Further, HK and low molecular mass kininogen, but not factor XII, blocked biotin-HK binding to cytokeratin, and peptides of each cell binding region of HK on domains 3,4, and 5 blocked biotin-HK binding to cytokeratin. gC1qR and soluble urokinase-like plasminogen activator receptor also inhibited biotin-HK binding to cytokeratin. These investigations identify a new function for cytokeratin 1 as a kininogen binding protein. Cytokeratins, members of the family of intermediate filament proteins, may represent a new class of receptors.
Figures


References
-
- Hong S L. Thromb Res. 1980;18:787–796. - PubMed
-
- Crutchley D J, Ryan J W, Ryan U S, Fisher G H. Biochim Biophys Acta. 1983;751:99–107. - PubMed
-
- Holland J A, Pritchard K A, Pappolla M A, Wolin R S, Rogers N J, Stemerman M B. J Cell Physiol. 1990;143:21–25. - PubMed
-
- Smith D, Gilbert M, Owen W G. Blood. 1983;66:835–839. - PubMed
-
- Brown N J, Nadeau J H, Vaughan D E. Thromb Haemostasis. 1997;77:522–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

