Overlapping roles and asymmetrical cross-regulation of the USF proteins in mice
- PMID: 9520440
- PMCID: PMC19910
- DOI: 10.1073/pnas.95.7.3758
Overlapping roles and asymmetrical cross-regulation of the USF proteins in mice
Abstract
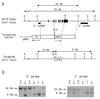
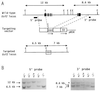
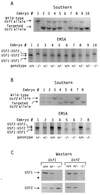
USF1 and USF2 are ubiquitously expressed transcription factors implicated as antagonists of the c-Myc protooncoprotein in the control of cellular proliferation. To determine the biological role of the USF proteins, mutant mice were generated by homologous recombination in embryonic stem cells. USF1-null mice were viable and fertile, with only slight behavioral abnormalities. However, these mice contained elevated levels of USF2, which may compensate for the absence of USF1. In contrast, USF2-null mice contained reduced levels of USF1 and displayed an obvious growth defect: they were 20-40% smaller at birth than their wild-type or heterozygous littermates and maintained a smaller size with proportionate features throughout postnatal development. Some of the USF-deficient mice, especially among the females, were prone to spontaneous epileptic seizures, suggesting that USF is important in normal brain function. Among the double mutants, an embryonic lethal phenotype was observed for mice that were homozygous for the Usf2 mutation and either heterozygous or homozygous for the Usf1 mutation, demonstrating that the USF proteins are essential in embryonic development.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

