Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner
- PMID: 9520470
- PMCID: PMC19940
- DOI: 10.1073/pnas.95.7.3931
Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner
Abstract
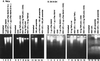
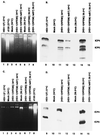
Several publications have attested to the ability of herpes simplex viruses to protect cells against apoptosis. We investigated the ability of the virus to protect cells in continuous cultivation from apoptosis induced by the virus itself, and by other known inducers such as exposure to the tumor necrosis factor alpha (TNFalpha), antibody to Fas, C2-ceramide, osmotic shock (sorbitol), and thermal shock. The salient features of the results were that the virus was able to protect cells against apoptosis by all of the agents tested, and that apoptosis induced by the virus was a very early event that did not require de novo expression of viral genes. However, these events were cell-type specific. Thus: (i) The cell lines tested exhibited fragmented chromosomal DNA following infection with a virus lacking functional alpha4 and US3 genes encoding the major regulatory protein and a viral protein kinase, respectively, but not by wild-type virus. (ii) Wild-type virus protected subconfluent SK-N-SH but not HeLa cells against induction of apoptosis by anti-Fas antibody, TNFalpha, C2-ceramide, and thermal shock. Confluent SK-N-SH cells were not protected from osmotic shock-induced apoptosis by wild-type infection. (iii) Wild-type virus protected SK-N-SH but not HeLa cells against induction of apoptosis by sorbitol, anti-Fas antibody, or TNFalpha and C2-ceramide. (iv) Mutant HSV-1(HFEM)tsB7 at the nonpermissive temperature infects cells but the DNA is not released from capsids, and therefore viral gene expression is restricted to the function of viral proteins introduced into the cell along with the capsid containing the viral DNA. HSV-1(HFEM)tsB7 induced apoptosis in Vero cells but not in SK-N-SH cells infected and maintained at 39.5 degrees C. (v) Tests of two caspase inhibitors showed that they blocked apoptosis induced by C2-ceramide and sorbitol, but were not able to block apoptosis induced by the virus lacking functional alpha4 and US3 genes. We conclude that HSV-1 triggers apoptosis at multiple metabolic checkpoints and in turn has evolved mechanisms to block apoptosis at each point and that some of the pathways of induction are shared with exogenous inducers tested in this study whereas others are not.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

