Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells
- PMID: 9520471
- PMCID: PMC19941
- DOI: 10.1073/pnas.95.7.3937
Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells
Abstract
Hematopoietic cells and their progenitors play important roles in human cytomegalovirus latency and reactivation. Latent infection has been evaluated in defined populations of myeloid-lineage-committed progenitor cells coexpressing CD33 and CD15 or CD33 and CD14 along with the dendritic cell markers CD1a and CD10. These CD33+ cell populations were found to support latency and expression of viral latency-associated transcripts and to undergo reactivation of productive viral replication when differentiated in the presence of human fibroblasts. Reactivation was also observed when myeloid cells were carried in the presence of fibroblast-conditioned medium or medium supplemented with certain cytokines (interferon gamma, tumor necrosis factor alpha, interleukin 4, or granulocyte-macrophage colony-simulating factor), suggesting that cell differentiation pathways act as determinants of reactivation. More primitive CD34+ hematopoietic cells were also found to be susceptible to viral infection and latency was maintained as these cells differentiated into CD33+-lineage-committed populations. Between 0.01% and 0.001% of CD33+ CD14+ or CD33+ CD15+ bone marrow mononuclear cells isolated from naturally infected individuals were found to express latent transcripts. Thus, cytomegalovirus is carried within a small percentage of myeloid and dendritic cell progenitors in the healthy seropositive host. Virus reactivation may be triggered by factors associated with the inflammatory response.
Figures


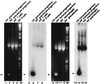
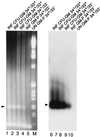
References
-
- Alford C A, Britt W J. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott-Raven; 1996. pp. 2493–2534.
-
- Maciejewski J P, Bruening E E, Donahue R E, Sellers S E, Carter C, Young N S, St. Jeor S. Virology. 1993;195:327–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

