Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons
- PMID: 9520484
- PMCID: PMC19954
- DOI: 10.1073/pnas.95.7.4013
Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons
Abstract
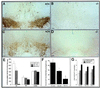
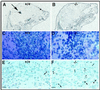
Nurr1 is a member of the nuclear receptor superfamily of transcription factors that is expressed predominantly in the central nervous system, including developing and mature dopaminergic neurons. Recent studies have demonstrated that Nurr1 is essential for the induction of phenotypic markers of ventral mid-brain dopaminergic neurons whose generation is specified by the floor plate-derived morphogenic signal sonic hedgehog (SHH), but the precise role of Nurr1 in this differentiative pathway has not been established. To provide further insights into the role of Nurr1 in the final differentiation pathway, we have examined the fate of dopamine cell precursors in Nurr1 null mutant mice. Here we demonstrate that Nurr1 functions at the later stages of dopamine cell development to drive differentiation of ventral mesencephalic late dopaminergic precursor neurons. In the absence of Nurr1, neuroepithelial cells that give rise to dopaminergic neurons adopt a normal ventral localization and neuronal phenotype characterized by expression of the homeodomain transcription factor and mesencephalic marker, Ptx-3, at embryonic day 11.5. However, these late precursors fail to induce a dopaminergic phenotype, indicating that Nurr1 is essential for specifying commitment of mesencephalic precursors to the full dopaminergic phenotype. Further, as development progresses, these mid-brain dopamine precursor cells degenerate in the absence of Nurr1, resulting in loss of Ptx-3 expression and a concomitant increase in apoptosis of ventral midbrain neurons in newborn null mutant mice. Taken together, these data indicate that Nurr1 is essential for both survival and final differentiation of ventral mesencephalic late dopaminergic precursor neurons into a complete dopaminergic phenotype.
Figures



References
-
- Bjorklund A, Lindvall O. In: Handbook of Chemical Neuroanatomy. Bjorklund A, Hokfelt T, editors. Amsterdam: Elsevier; 1984. pp. 55–122.
-
- Lindvall O, Bjorlund A. In: Chemical Neuroanatomy. Emson P C, editor. New York: Raven; 1983. pp. 229–255.
-
- Hirsch E C, Graybiel A M, Agid Y A. Nature (London) 1988;334:345–348. - PubMed
-
- Self D W, Nestler E J. Annu Rev Neurosci. 1995;18:463–495. - PubMed
-
- Seeman P, Guan H C, Van Tol H H M. Nature (London) 1997;365:441–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

