Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara- clinical isolates
- PMID: 9521147
- PMCID: PMC121362
- DOI: 10.1128/CDLI.5.2.225-229.1998
Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara- clinical isolates
Abstract
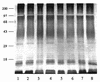
Different lines of evidence suggest that a discrepancy between the distribution of Burkholderia (Pseudomonas) pseudomallei in the environment and the distribution of the disease melioidosis is attributable, at least in part, to phenotypic differences between clinical and some environmental isolates. Two antigenically and biochemically distinct biotypes have been described, only one of which is virulent. In this study, lipopolysaccharides (LPSs) were extracted by the proteinase K digestion method from a total of 214 B. pseudomallei isolates, and their immunoreactivities with sera from patients with different clinical spectra and with other infections were evaluated. With the exception of4 isolates from a total of 214 tested, the sodium dodecyl sulfate-polyacrylamide gel electrophoresis silver-staining profiles of the LPSs from the two biotypes showed identical ladder patterns that were typical for smooth LPSs from other gram-negative bacteria. The 210 isolates with typical LPS patterns (119 Ara- clinical, 13 Ara- soil, 70 Ara+ soil, and 8 reference National Type Culture Collection strains) also exhibited similar immunoblot profiles against pooled sera from patients with melioidosis and hyperimmune mouse sera. Concordant findings were noted in the indirect enzyme-linked immunosorbent assay with Ara- and Ara+ LPSs to coat the microtiter plates. The LPSs of the different B. pseudomallei biotypes appear antigenically indistinguishable. It is, therefore, unlikely that this component is related to the virulence and pathogenicity of B. pseudomallei.
Figures


References
-
- Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major course of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

