The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication
- PMID: 9525637
- PMCID: PMC109762
- DOI: 10.1128/JVI.72.4.3117-3128.1998
The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication
Abstract
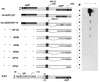
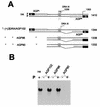
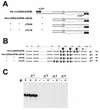
The paramyxovirus genome, a nonsegmented, negative-polarity, single-stranded RNA of approximately 15 kb, contains six transcription units flanked at the 3' and 5' ends by a short (approximately 50- to 60-nucleotide) extracistronic sequence, dubbed the positive and negative leader regions. These leader template regions, present at the 3' end of the genome and the antigenome, have been shown to contain essential signals governing RNA replication activity. Whether they are sufficient to promote replication is still open to question. By using a series of Sendai virus defective interfering RNAs carrying a nested set of deletions in the promoter regions, it is shown here that for both the genomic and antigenomic promoters, a 3'-end RNA sequence of 96 nucleotides is required to allow replication. Sequence comparison of active and inactive promoters led to the identification of a set of three nucleotide hexamers (nucleotides 79 to 84, 85 to 90, and 91 to 96) containing a repeated motif RXXYXX [shown as 5'-3' positive-strand]. Sequential mutation of each hexamer into its complementary sequence confirmed their essential role. The three hexamers are required, and their relative positioning is important, since displacing them by 6 nucleotides destroyed promoter function. RNAs carrying degenerate nucleotides in the three hexamers were used as replication templates. They led to the selection of actively replicating RNA species exclusively carrying the basic motif (GNNNNN)3 from nucleotides 79 to 96. These results clearly show that, apart from the region from nucleotides 1 to 31, previously identified as governing Sendai virus replication activity, a second element, spanning at the most nucleotides 79 to 96, appears essential. Thus, the paramyxovirus replication promoters are not confined to the leader template regions, as seems to be the case for the rhabdoviruses.
Figures







References
-
- Blumberg B M, Chan J, Udem S A. Function of paramyxovirus 3′ and 5′ end sequences in theory and practice. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Publishing Corp.; 1991. pp. 235–247.
-
- Blumberg B M, Giorgi C, Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983;32:559–567. - PubMed
-
- Blumberg B M, Leppert M, Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981;23:837–845. - PubMed
-
- Calain P, Curran J, Kolakofsky D, Roux L. Molecular cloning of natural paramyxovirus copy-back defective interfering RNAs and their expression from DNA. Virology. 1992;191:62–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

