Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS
- PMID: 9525998
- PMCID: PMC6792589
- DOI: 10.1523/JNEUROSCI.18-08-02808.1998
Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS
Abstract
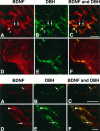
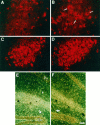
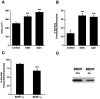
In this report, we have tested the hypothesis that brain-derived neurotrophic factor (BDNF) is an anterograde neurotrophic factor in the CNS and have focused on central noradrenergic neurons that synthesize BDNF. Double-label immunocytochemistry for BDNF and dopamine-beta-hydroxylase (DBH), a marker for noradrenergic neurons, demonstrated that BDNF is partially localized to noradrenergic nerve fibers and terminals in the adult rat brain. To test the functional importance of this anterograde BDNF, we analyzed transgenic mice carrying a DBH-BDNF minigene. Increased synthesis of BDNF in noradrenergic neurons of DBH-BDNF mice caused elevated TrkB tyrosine kinase activation throughout postnatal life in the neocortex, a noradrenergic target region. This afferently regulated increase in TrkB receptor activity led to long-lasting alterations in cortical morphology. To determine whether noradrenergic neuron-expressed BDNF also anterogradely regulated neuronal survival, we examined a second noradrenergic target, neonatal facial motoneurons. One week after axotomy, 72% of facial motoneurons were lost in control animals, whereas only 30-35% were lost in DBH-BDNF transgenic mice. Altogether, these results indicate that BDNF is anterogradely transported to fibers and terminals of noradrenergic neurons, that anterogradely secreted BDNF causes activation of TrkB in target regions, and that this secretion has functional consequences for target neuron survival and differentiation. This presynaptic secretion of BDNF may provide a cellular mechanism for modulating neural circuitry, in either the developing or mature nervous system.
Figures


References
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay R, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
-
- Altman J, Bayer SA. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp Neurol. 1990;107:23–35. - PubMed
-
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. - PubMed
-
- Bayer SA, Altman J. Neurogenesis and neuronal migration. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1994. pp. 1041–1078.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
