Contributions of the optic tectum and the retina as sources of brain-derived neurotrophic factor for retinal ganglion cells in the chick embryo
- PMID: 9526006
- PMCID: PMC6792593
- DOI: 10.1523/JNEUROSCI.18-08-02891.1998
Contributions of the optic tectum and the retina as sources of brain-derived neurotrophic factor for retinal ganglion cells in the chick embryo
Abstract
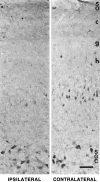
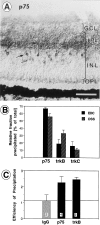
Retinal ganglion cells (RGC) are supported by brain-derived neurotrophic factor (BDNF), but it is not known if BDNF acts as a target-derived factor or as an afferent or autocrine trophic factor. Here we demonstrate that BDNF mRNA is expressed in the retinorecipient layer of the chick optic tectum as well as in the inner nuclear layer and ganglion cell layer of the retina. Amacrine cells rather than RGC were the main source of BDNF mRNA in the ganglion cell layer, as determined by in situ hybridization that was combined with retrograde labeling of RGC and destruction of RGC by optic stalk transection, followed by quantitative RT-PCR. Cells in the ganglion cell layer as well as the retinorecipient layers of the optic tectum were BDNF-immunolabeled. After injections into the tectum, radio-iodinated BDNF was transported to the retina where autoradiographic label accumulated in the inner plexiform and ganglion cell layers. After intraocular injection, iodinated BDNF accumulated in these same retinal layers and correlated with the distribution of p75 neurotrophin receptor protein. The majority of cross-linked receptor-bound BDNF in the retina immunoprecipitated with p75 antibodies. No difference in the intensity of BDNF immunolabel was observed in the experimental retina or tectum after optic stalk transection, indicating that most of the BDNF in the RGC was not derived from the optic tectum. These data indicate that a substantial fraction of the BDNF in the ganglion cell layer is derived from local sources, afferents within the retina, rather than from the optic tectum via retrograde transport.
Figures








References
-
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
-
- AryPires R, Nakatani M, Rehen SK, Linden R. Developmentally regulated release of intraretinal neurotrophic factors in vitro. Int J Dev Neurosci. 1997;15:239–255. - PubMed
-
- Auerbach R, Kubal L, Folkman J. A simple procedure for the long-term cultivation of chick embryos. Dev Biol. 1974;41:391–394. - PubMed
-
- Ballarin M, Ernfors N, Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991;114:35–43. - PubMed
-
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
