A Ca2+-independent receptor for alpha-latrotoxin, CIRL, mediates effects on secretion via multiple mechanisms
- PMID: 9526008
- PMCID: PMC6792605
- DOI: 10.1523/JNEUROSCI.18-08-02914.1998
A Ca2+-independent receptor for alpha-latrotoxin, CIRL, mediates effects on secretion via multiple mechanisms
Abstract
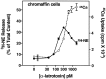
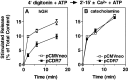
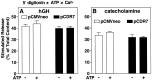
alpha-Latrotoxin (alpha-Ltx), a component of black widow spider venom, stimulates secretion from nerve terminals and from PC12 cells. In this study we examine the effects of expression of a newly cloned Ca2+-independent receptor for alpha-Ltx (CIRL) on secretion from bovine chromaffin cells. We first characterized the effect of alpha-Ltx on secretion from untransfected cells. alpha-Ltx, by binding in a Ca2+-independent manner to an endogenous receptor, causes subsequent Ca2+-dependent secretion from intact cells. The stimulation of secretion is correlated with Ca2+ influx caused by the toxin. In permeabilized cells in which the Ca2+ concentration is regulated by buffer, alpha-Ltx also enhances Ca2+-dependent secretion, indicating a direct role of the endogenous receptor in the secretory pathway. Expression of CIRL increased the sensitivity of intact and permeabilized cells to the effects of alpha-Ltx, demonstrating that this protein is functional in coupling to secretion. Importantly, in the absence of alpha-Ltx, the expression of CIRL specifically inhibited the ATP-dependent component of secretion in permeabilized cells without affecting the ATP-independent secretion. This suggests that this receptor modulates the normal function of the regulated secretory pathway and that alpha-Ltx may act by reversing the inhibitory effects of the receptor.
Figures







References
-
- Barnett DW, Liu J, Misler S. Single-cell measurements of quantal secretion induced by α-latrotoxin from rat adrenal chromaffin cells: dependence on extracellular Ca2+. Pflügers Arch. 1996;432:1039–1046. - PubMed
-
- Bittner MA, Holz RW. A temperature-sensitive step in exocytosis. J Biol Chem. 1992a;267:16226–16229. - PubMed
-
- Bittner MA, Holz RW. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992b;267:16219–16225. - PubMed
-
- Bittner MA, Bennett MK, Holz RW. Evidence that syntaxin 1A is involved in storage in the secretory pathway. J Biol Chem. 1996;271:11214–11221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
