Evidence for a tetrameric structure of recombinant NMDA receptors
- PMID: 9526012
- PMCID: PMC6792599
- DOI: 10.1523/JNEUROSCI.18-08-02954.1998
Evidence for a tetrameric structure of recombinant NMDA receptors
Abstract
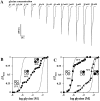
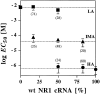
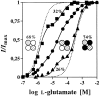
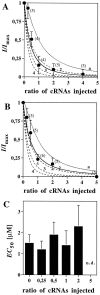
The amino acids L-glutamate and glycine are essential agonists of the excitatory NMDA receptor, a subtype of the ionotropic glutamate receptor family. The native NMDA receptor is composed of two types of homologous membrane-spanning subunits, NR1 and NR2. Here, the numbers of glycine-binding NR1 and glutamate-binding NR2 subunits in the NMDA receptor hetero-oligomer were determined by coexpressing the wild-type (wt) NR1 with the low-affinity mutant NR1(Q387K), and the wt NR2B with the low-affinity mutant NR2BE387A, subunits in Xenopus oocytes. In both cases, analysis of the resulting dose-response curves revealed three independent components of glycine and glutamate sensitivity. These correspond to the respective wild-type and mutant affinities and an additional intermediate hybrid affinity, indicating the existence of three discrete receptor populations. Binomial analysis of these data indicates the presence of two glycine and two glutamate binding subunits in the functional receptor. In addition, we analyzed the inhibitory effects of the negative dominant NR1(R505K) and NR2BR493K mutants on maximal inducible whole-cell currents of wt NR1/NR2B receptors. The inhibition profiles obtained on expression of increasing amounts of these mutant proteins again were fitted best by assuming an incorporation of two NR1 and two NR2 subunits into the receptor hetero-oligomer. Our data are consistent with NMDA receptors being tetrameric proteins that are composed of four homologous subunits.
Figures
References
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
-
- Béhé P, Stern P, Wyllie D, Nasser M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc R Soc Lond B Biol Sci. 1995;262:205–213. - PubMed
-
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990;29:3591–3599. - PubMed
-
- Blackstone CD, Moss SJ, Martin LJ, Huganir R. Biochemical characterization and localization of a non-NMDA receptor in rat brain. J Neurochem. 1992;58:1118–1126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
