Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells
- PMID: 9528750
- PMCID: PMC121408
- DOI: 10.1128/MCB.18.4.1783
Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells
Abstract
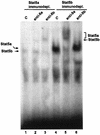
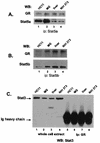
The lactogenic hormones, i.e., prolactin and glucocorticoids, act in concert to stimulate transcription factors responsible for hormone-dependent milk protein gene expression. In the mammary gland, prolactin activates Stat5a and Stat5b and glucocorticoids activate the glucocorticoid receptor (GR). Immunoprecipitation experiments revealed that in mammary cells, Stat5a, Stat5b, and the GR are physically associated in vivo. The association is not dependent on lactogenic hormone treatment and is evident at all stages of mammary gland development. Immunodepletion experiments indicated that a fraction of GR and Stat5 proteins are not associated, suggesting that there are different intracellular pools of these proteins. Lactogenic hormone treatment of HC11 mammary cells resulted in tyrosine phosphorylation of Stat5a and Stat5b, dimerization, and rapid nuclear translocation of both Stat5 proteins. Following hormone treatment, Stat5a-Stat5b heterodimers were detected by their coimmunoprecipitation. In addition, immunodepletion experiments followed by gel shift analyses revealed the presence of active Stat5a and Stat5b homodimers. In mammary cells, Stat5b homodimers are less abundant than Stat5a homodimers. Although the GR does not bind the Stat5 DNA binding site directly, it could be detected with the Stat5-DNA complex. These results suggest that glucocorticoids affect milk protein gene expression via association of the GR with Stat5. Thus, there is a functional coupling between Stat-dependent and nuclear hormone receptor-dependent gene transcription.
Figures




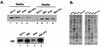


Similar articles
-
Lactogenic hormone activation of Stat5 and transcription of the beta-casein gene in mammary epithelial cells is independent of p42 ERK2 mitogen-activated protein kinase activity.J Biol Chem. 1996 Dec 13;271(50):31863-8. doi: 10.1074/jbc.271.50.31863. J Biol Chem. 1996. PMID: 8943229
-
Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation.Mol Endocrinol. 1996 Dec;10(12):1496-506. doi: 10.1210/mend.10.12.8961260. Mol Endocrinol. 1996. PMID: 8961260
-
A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b.J Biol Chem. 2000 Dec 15;275(50):39718-26. doi: 10.1074/jbc.M005615200. J Biol Chem. 2000. Retraction in: J Biol Chem. 2011 Mar 25;286(12):10888. doi: 10.1074/jbc.a110.005615. PMID: 10993888 Retracted.
-
Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation.Cytokine Growth Factor Rev. 1999 Jun;10(2):131-57. doi: 10.1016/s1359-6101(99)00011-8. Cytokine Growth Factor Rev. 1999. PMID: 10743504 Review.
-
Growth hormone pulse-activated STAT5 signalling: a unique regulatory mechanism governing sexual dimorphism of liver gene expression.Novartis Found Symp. 2000;227:61-74; discussion 75-81. doi: 10.1002/0470870796.ch5. Novartis Found Symp. 2000. PMID: 10752065 Review.
Cited by
-
Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer.Mol Cell Endocrinol. 2014 Jan 25;382(1):616-621. doi: 10.1016/j.mce.2013.03.010. Epub 2013 Mar 24. Mol Cell Endocrinol. 2014. PMID: 23531638 Free PMC article. Review.
-
Janus kinases and signal transducers and activators of transcription: their roles in cytokine signaling, development and immunoregulation.Arthritis Res. 2000;2(1):16-32. doi: 10.1186/ar66. Epub 1999 Dec 23. Arthritis Res. 2000. PMID: 11094415 Free PMC article. Review.
-
Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38.Mol Cell Biol. 2002 Nov;22(22):7802-11. doi: 10.1128/MCB.22.22.7802-7811.2002. Mol Cell Biol. 2002. PMID: 12391149 Free PMC article.
-
Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching.J Clin Invest. 2001 Feb;107(3):371-8. doi: 10.1172/JCI10168. J Clin Invest. 2001. PMID: 11160161 Free PMC article.
-
Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms.Mol Endocrinol. 2011 Jul;25(7):1075-86. doi: 10.1210/me.2011-0068. Epub 2011 Apr 21. Mol Endocrinol. 2011. PMID: 21511881 Free PMC article. Review.
References
-
- Alonso C R, Pesce C G, Kornblihtt A R. The CCAAT-binding proteins CP1 and NF-I cooperate with ATF-2 in the transcription of the fibronectin gene. J Biol Chem. 1996;271:22271–22279. - PubMed
-
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
-
- Cella, N. Unpublished data.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
