Interactions within the yeast Sm core complex: from proteins to amino acids
- PMID: 9528767
- PMCID: PMC121425
- DOI: 10.1128/MCB.18.4.1956
Interactions within the yeast Sm core complex: from proteins to amino acids
Abstract
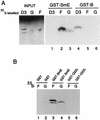
Sm core proteins play an essential role in the formation of small nuclear ribonucleoprotein particles (snRNPs) by binding to small nuclear RNAs and participating in a network of protein interactions. The two-hybrid system was used to identify SmE interacting proteins and to test for interactions between all pairwise combinations of yeast Sm proteins. We observed interactions between SmB and SmD3, SmE and SmF, and SmE and SmG. For these interactions, a direct biochemical assay confirmed the validity of the results obtained in vivo. To map the protein-protein interaction surface of Sm proteins, we generated a library of SmE mutants and investigated their ability to interact with SmF and/or SmG proteins in the two-hybrid system. Several classes of mutants were observed: some mutants are unable to interact with either SmF or SmG proteins, some interact with SmG but not with SmF, while others interact moderately with SmF but not with SmG. Our mutational analysis of yeast SmE protein shows that conserved hydrophobic residues are essential for interactions with SmF and SmG as well as for viability. Surprisingly, we observed that other evolutionarily conserved positions are tolerant to mutations, with substitutions affecting binding to SmF and SmG only mildly and conferring a wild-type growth phenotype.
Figures


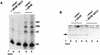


References
-
- Altschul S F, Gish W, Miller W, Meyer E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bordonné R, Banroques J, Abelson J, Guthrie C. Domains of yeast U4 spliceosomal RNA required for PRP4 protein binding, snRNP-snRNP interactions, and pre-mRNA splicing in vivo. Genes Dev. 1990;4:1185–1196. - PubMed
-
- Bordonné R, Tarassov I. The yeast SME1 gene encodes the homologue of human E core protein. Gene. 1996;176:111–117. - PubMed
-
- Breeden L, Nasmyth K. Regulation of HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
