A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1
- PMID: 9528787
- PMCID: PMC121452
- DOI: 10.1128/MCB.18.4.2153
A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1
Abstract
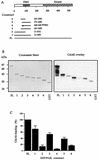
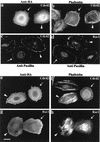
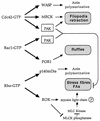
AlphaPAK in a constitutively active form can exert morphological effects (E. Manser, H.-Y. Huang, T.-H. Loo, X.-Q. Chen, J.-M. Dong, T. Leung, and L. Lim, Mol. Cell. Biol. 17:1129-1143, 1997) resembling those of Cdc42G12V. PAK family kinases, conserved from yeasts to humans, are directly activated by Cdc42 or Rac1 through interaction with a conserved N-terminal motif (corresponding to residues 71 to 137 in alphaPAK). alphaPAK mutants with substitutions in this motif that resulted in severely reduced Cdc42 binding can be recruited normally to Cdc42G12V-driven focal complexes. Mutation of residues in the C-terminal portion of the motif (residues 101 to 137), though not affecting Cdc42 binding, produced a constitutively active kinase, suggesting this to be a negative regulatory region. Indeed, a 67-residue polypeptide encoding alphaPAK83-149 potently inhibited GTPgammaS-bound Cdc42-mediated kinase activation of both alphaPAK and betaPAK. Coexpression of this PAK inhibitor with Cdc42G12V prevented the formation of peripheral actin microspikes and associated loss of stress fibers normally induced by the p21. Coexpression of PAK inhibitor with Rac1G12V also prevented loss of stress fibers but not ruffling induced by the p21. Coexpression of alphaPAK83-149 completely blocked the phenotypic effects of hyperactive alphaPAKL107F in promoting dissolution of focal adhesions and actin stress fibers. These results, coupled with previous observations with constitutively active PAK, demonstrate that these kinases play an important role downstream of Cdc42 and Rac1 in cytoskeletal reorganization.
Figures





References
-
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1995;6:70–75. - PubMed
-
- Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signalling leads to JNK and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
-
- Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21cdc42/rac activated kinase. J Biol Chem. 1995;270:22731–22737. - PubMed
-
- Benner G E, Dennis P B, Masaracchia R A. Activation of an S6/H4 kinase (PAK 65) from human placenta by intramolecular and intermolecular autophosphorylation. J Biol Chem. 1995;270:21121–21128. - PubMed
-
- Bokoch G M, Wang Y, Bohl B P, Sells M A, Quilliam L A, Knaus U G. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
