Shc and Enigma are both required for mitogenic signaling by Ret/ptc2
- PMID: 9528800
- PMCID: PMC121481
- DOI: 10.1128/MCB.18.4.2298
Shc and Enigma are both required for mitogenic signaling by Ret/ptc2
Abstract
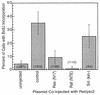
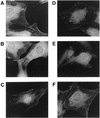
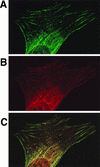
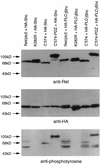
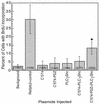
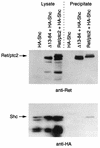
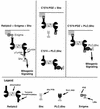
Ret/ptc2 is a constitutively active, oncogenic form of the c-Ret receptor tyrosine kinase. Like the other papillary thyroid carcinoma forms of Ret, Ret/ptc2 is activated through fusion of the Ret tyrosine kinase domain to the dimerization domain of another protein. Investigation of requirements for Ret/ptc2 mitogenic activity, using coexpression with dominant negative forms of Ras and Raf, indicated that these proteins are required for mitogenic signaling by Ret/ptc2. Because activation of Ras requires recruitment of Grb2 and SOS to the plasma membrane, the subcellular distribution of Ret/ptc2 was investigated, and it was found to localize to the cell periphery. This localization was mediated by association with Enigma via the Ret/ptc2 sequence containing tyrosine 586. Because Shc interacts with MEN2 forms of Ret, and because phosphorylation of Shc results in Grb2 recruitment and subsequent signaling through Ras and Raf, the potential interaction between Ret/ptc2 and Shc was investigated. The PTB domain of Shc also interacted with Ret/ptc2 at tyrosine 586, and this association resulted in tyrosine phosphorylation of Shc. Coexpression of chimeric proteins demonstrated that mitogenic signaling from Ret/ptc2 required both recruitment of Shc and subcellular localization by Enigma. Because Shc and Enigma interact with the same site on a Ret/ptc2 monomer, dimerization of Ret/ptc2 allows assembly of molecular complexes that are properly localized via Enigma and transmit mitogenic signals via Shc.
Figures




References
-
- Asai N, Murakami H, Iwashita T, Takahashi M. A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with Shc adaptor proteins. J Biol Chem. 1996;271:17644–17649. - PubMed
-
- Attie T, Pelet A, Sarda P, Edery P, Eng C, Mulligan L M, Ponder B A J, Munnich A, Lyonnet S. A 7bp deletion of the RET proto-oncogene mutations in familial Hirschsprung’s disease. Hum Mol Genet. 1994;3:1439–1440. - PubMed
-
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Bongarzone I, Butti M G, Coronelli S, Borrello M G, Santoro M, Mondellini P, Pilotti S, Fusco A, Della Porta G, Pierotti M A. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 1994;54:2979–2985. - PubMed
-
- Bongarzone I, Monzini N, Borrello M G, Carcano C, Ferraresi G, Arighi E, Mondellini P, Della Porta G, Pierotti M A. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RIα of cyclic AMP-dependent protein kinase A. Mol Cell Biol. 1993;13:358–366. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
