Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines
- PMID: 9528802
- PMCID: PMC121486
- DOI: 10.1128/MCB.18.4.2324
Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines
Abstract
We have compared the ability of two mammalian Notch homologs, mouse Notchl and Notch2, to inhibit the granulocytic differentiation of 32D myeloid progenitor cells. 32D cells undergo granulocytic differentiation when stimulated with either granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF). Expression of the activated intracellular domain of Notch1 inhibits the differentiation induced by G-CSF but not by GM-CSF; conversely, the corresponding domain of Notch2 inhibits differentiation in response to GM-CSF but not to G-CSF. The region immediately C-terminal to the cdc10 domain of Notch confers cytokine specificity on the cdc10 domain. The cytokine response patterns of Notch1 and Notch2 are transferred with this region, which we have termed the Notch cytokine response (NCR) region. The NCR region is also associated with differences in posttranslational modification and subcellular localization of the different Notch molecules. These findings suggest that the multiple forms of Notch found in mammals have structural differences that allow their function to be modulated by specific differentiation signals.
Figures



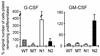




References
-
- Artavanis-Tsakonas S, Matsuno K, Fortini M E. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Artavanis-Tsakonas S, Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991;7:403–408. - PubMed
-
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. - PubMed
-
- Cagan R L, Ready D F. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. - PubMed
-
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
