Human matrix attachment regions insulate transgene expression from chromosomal position effects in Drosophila melanogaster
- PMID: 9528807
- PMCID: PMC121496
- DOI: 10.1128/MCB.18.4.2382
Human matrix attachment regions insulate transgene expression from chromosomal position effects in Drosophila melanogaster
Abstract
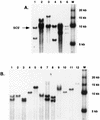
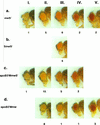
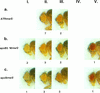
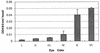
Germ line transformation of white- Drosophila embryos with P-element vectors containing white expression cassettes results in flies with different eye color phenotypes due to position effects at the sites of transgene insertion. These position effects can be cured by specific DNA elements, such as the Drosophila scs and scs' elements, that have insulator activity in vivo. We have used this system to determine whether human matrix attachment regions (MARs) can function as insulator elements in vivo. Two different human MARs, from the apolipoprotein B and alpha1-antitrypsin loci, insulated white transgene expression from position effects in Drosophila melanogaster. Both elements reduced variability in transgene expression without enhancing levels of white gene expression. In contrast, expression of white transgenes containing human DNA segments without matrix-binding activity was highly variable in Drosophila transformants. These data indicate that human MARs can function as insulator elements in vivo.
Figures


Similar articles
-
Human matrix attachment regions are necessary for the establishment but not the maintenance of transgene insulation in Drosophila melanogaster.Mol Cell Biol. 2004 Dec;24(23):10236-45. doi: 10.1128/MCB.24.23.10236-10245.2004. Mol Cell Biol. 2004. PMID: 15542833 Free PMC article.
-
[The role of chromosomal regions anchored to the nuclear envelope in the functional organization of chromosomes].Genetika. 2010 Sep;46(9):1175-7. Genetika. 2010. PMID: 21061611 Russian.
-
Drosophila mini-white model system: new insights into positive position effects and the role of transcriptional terminators and gypsy insulator in transgene shielding.Nucleic Acids Res. 2010 Jan;38(1):39-47. doi: 10.1093/nar/gkp877. Epub 2009 Oct 23. Nucleic Acids Res. 2010. PMID: 19854952 Free PMC article.
-
Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophila heterochromatin.Chromosoma. 1998 Nov;107(5):277-85. doi: 10.1007/s004120050309. Chromosoma. 1998. PMID: 9880760
-
Use of matrix attachment regions (MARs) to minimize transgene silencing.Plant Mol Biol. 2000 Jun;43(2-3):361-76. doi: 10.1023/a:1006424621037. Plant Mol Biol. 2000. PMID: 10999416 Review.
Cited by
-
Enhancer blocking by the Drosophila gypsy insulator depends upon insulator anatomy and enhancer strength.Genetics. 1999 Oct;153(2):787-98. doi: 10.1093/genetics/153.2.787. Genetics. 1999. PMID: 10511558 Free PMC article.
-
Minimizing the unpredictability of transgene expression in plants: the role of genetic insulators.Plant Cell Rep. 2012 Jan;31(1):13-25. doi: 10.1007/s00299-011-1167-y. Epub 2011 Oct 11. Plant Cell Rep. 2012. PMID: 21987122 Review.
-
Current advances in retroviral gene therapy.Curr Gene Ther. 2011 Jun;11(3):218-28. doi: 10.2174/156652311795684740. Curr Gene Ther. 2011. PMID: 21453283 Free PMC article. Review.
-
Identification of a potent MAR element from the human genome and assessment of its activity in stably transfected CHO cells.J Cell Mol Med. 2018 Feb;22(2):1095-1102. doi: 10.1111/jcmm.13361. Epub 2017 Oct 27. J Cell Mol Med. 2018. PMID: 29077269 Free PMC article.
-
Position-independent expression of transgenes in zebrafish.Transgenic Res. 1999 Oct;8(5):321-34. doi: 10.1023/a:1008993022331. Transgenic Res. 1999. PMID: 10669943
References
-
- Bao J J, Reed-Fourquet L, Sifers R N, Kidd V J, Woo S L. Molecular structure and sequence homology of a gene related to α1-antitrypsin in the human genome. Genomics. 1988;2:165–173. - PubMed
-
- Blackhart B D, Ludwig E M, Pierotti V R, Caiati L, Onasch M A, Wallis S C, Powell L, Pease R, Knott T J, Chu M L, et al. Structure of the human apolipoprotein B gene. J Biol Chem. 1986;261:15364–15367. - PubMed
-
- Bode J, Maass K. Chromatin domain surrounding the human interferon-β gene as defined by scaffold-attached regions. Biochemistry. 1988;27:4706–4711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
