Superoxide dismutase-dependent, catalase-sensitive peroxides in human endothelial cells infected by Rickettsia rickettsii
- PMID: 9529045
- PMCID: PMC108052
- DOI: 10.1128/IAI.66.4.1293-1298.1998
Superoxide dismutase-dependent, catalase-sensitive peroxides in human endothelial cells infected by Rickettsia rickettsii
Abstract
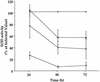
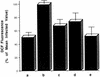
The generation and intracellular accumulation of reactive oxygen species have been shown to be associated with the infection of human umbilical vein endothelial cells (HUVEC) by Rickettsia rickettsii. In response to the oxidant superoxide, the activity of the enzyme superoxide dismutase (SOD) increases following infection by this obligate intracellular bacterium. Other oxidants which are capable of oxidizing the fluorescent probe 2',7'-dichlorofluorescin (DCFH) also accumulate intracellularly within infected cells. In the study reported here, we show that (i) an inhibitor of SOD, diethyldithiocarbamic acid, reduces the observed rise in SOD activity in infected cells by 40 to 60% and at the same time reduces the degree of intracellular oxidation of DCFH; (ii) catalase-sensitive peroxides can be detected in supernatants of R. rickettsii-infected cells shortly after rickettsial exposure; and (iii) fluorescence-activated cell sorter analysis demonstrates significant intracellular oxidant activity in infected cells within 5 h after exposure to R. rickettsii. The results of these experiments indicate that hydrogen peroxide is a major oxidant associated with infection of HUVEC by R. rickettsii and that intracellular oxidant activity sensitive to SOD inhibition is detectable early and prior to significant rickettsial multiplication and much earlier than the ultrastructural manifestations of cell injury seen by electron microscopy.
Figures


References
-
- Adams J S, Walker D H. The liver in Rocky Mountain spotted fever. Am J Clin Pathol. 1981;75:156–161. - PubMed
-
- Carter W O, Narayanan P K, Robinson J P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukocyte Biol. 1993;55:253–258. - PubMed
-
- Cathcart R, Schwiers E, Ames B N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134:111–116. - PubMed
-
- Eremeeva, M. E., and D. J. Silverman. Submitted for publication.
-
- Gimbrone M A., Jr Culture of vascular endothelium. Prog Hemostasis Thromb. 1976;3:1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

