M protein of the group A Streptococcus binds to the seventh short consensus repeat of human complement factor H
- PMID: 9529063
- PMCID: PMC108070
- DOI: 10.1128/IAI.66.4.1427-1431.1998
M protein of the group A Streptococcus binds to the seventh short consensus repeat of human complement factor H
Abstract
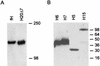
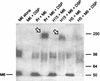
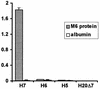
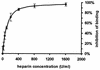
Streptococcus pyogenes evades complement by binding the complement-regulatory protein factor H (fH) via the central conserved C-repeat region of M protein. However, the corresponding binding region within fH has not previously been precisely localized. fH is composed of 20 conserved modules called short consensus repeats (SCRs), each of which contains approximately 60 amino acids. A series of fH truncated and deletion mutants were prepared, and their interaction with M6 protein was examined. The M protein binding site was initially localized to SCRs 6 to 15 as demonstrated by ligand dot blotting, chemical cross-linking, and enzyme-linked immunosorbent assay. SCR 7 was then shown to contain the M protein binding site, as a construct consisting of the first seven SCRs bound M protein but a construct containing the first six SCRs did not bind. In addition, deletion of SCR 7 from full-length fH abolished binding to M protein. SCR 7 is known to contain a heparin binding domain, and binding of fH to M6 protein was almost totally inhibited in the presence of 400 U of heparin per ml. These results localize the M6 protein binding site of fH to SCR 7 and indicate that it is in close proximity to the heparin binding site.
Figures
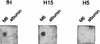
References
-
- Avery V M, Gordon D L. Characterization of factor H binding to human polymorphonuclear leukocytes. J Immunol. 1993;151:5545–5553. - PubMed
-
- Barlow P N, Campbell I D. Strategy for studying modular proteins: application to complement modules. Methods Enzymol. 1994;239:465–485. - PubMed
-
- Bisno A L, Stevens D L. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. - PubMed
-
- Blackmore T K, Hellwage J, Zipfel P F, Gordon D L. Identification of an additional heparin binding domain in the C-terminal region of human factor H. Exp Clin Immunogenet. 1997;14:30.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

