Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle
- PMID: 9529064
- PMCID: PMC108071
- DOI: 10.1128/IAI.66.4.1432-1438.1998
Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle
Abstract
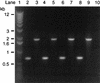
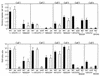
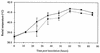
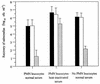
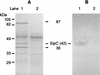
The induction of secretory and inflammatory responses in calves by Salmonella typhimurium and Salmonella dublin strains was compared, and the effects of mutations in the invH and stn genes were assessed. S. typhimurium induced greater secretory and inflammatory responses than S. dublin in bovine ileal loops, despite the fact that these serotypes were recovered from bovine ileal mucosa in comparable numbers (P. R. Watson, S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis, Infect. Immun. 63:2743-2754, 1995). These results implicate serotype-specific factors other than, or in addition to, intestinal invasion in the induction of enteritis. The secretory and inflammatory responses induced by S. typhimurium and S. dublin in bovine ligated ileal loops were not significantly altered by mutation of stn, which suggests that stn does not have a major role in Salmonella-induced enteritis. The invH mutation significantly reduced the secretory and inflammatory responses induced in bovine ileal loops, and this correlated with a reduction in the severity of enteritis following oral inoculation of calves. The attenuation associated with the invH mutation did not appear to be due to an increased susceptibility to the innate host defense mechanisms, because the resistance of S. typhimurium to the bactericidal action of either bovine polymorphonuclear leukocytes or bovine serum was not significantly altered. However, lysis of macrophages following infection with S. typhimurium was significantly reduced by the invH mutation. The invH mutation prevented the normal secretion of several proteins, including SipC, by S. typhimurium, indicating that the function of the inv-spa-encoded type III protein secretion system was disrupted. Taken together, these observations implicate inv-spa-dependent effectors in mediation of Salmonella-induced enteritis in cattle. Clearly, however, other undefined serotype-specific virulence factors are also involved in Salmonella-induced enteritis.
Figures




References
-
- Altmeyer R M, McNern J K, Bossio J C, Rosenshine I, Finlay B B, Galán J E. Cloning and molecular characterization of a gene involved in Salmonellaadherence and invasion of cultured epithelial cells. Mol Microbiol. 1993;7:89–98. - PubMed
-
- Barrow, P. (Institute for Animal Health). 1997. Personal communication.
-
- Carlson G P, Kaneko J J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973;142:853–855. - PubMed
-
- Chen L M, Kaniga K, Galán J E. Salmonellaspp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

