Characterization of anticapsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule
- PMID: 9529079
- PMCID: PMC108086
- DOI: 10.1128/IAI.66.4.1538-1546.1998
Characterization of anticapsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule
Abstract
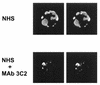
Incubation of the encapsulated yeast Cryptococcus neoformans in human serum leads to alternative pathway-mediated deposition of C3 fragments in the capsule. We examined the ability of monoclonal antibodies (MAbs) specific for different epitopes of the major capsular polysaccharide to alter the kinetics for classical and alternative pathway-mediated deposition of C3 onto a serotype A strain. We studied MAbs reactive with capsular serotypes A, B, C, and D (MAb group II); serotypes A, B, and D (MAb group III); and serotypes A and D (MAb group IV). The MAb groupings are based on antibody variable region usage which determines the antibody molecular structure. When both the classical and alternative pathways were operative, group II MAbs induced early classical pathway-mediated binding of C3 but reduced the overall rate of C3 accumulation and the amount of bound C3. Group III MAbs closely mimicked the effects of group II MAbs but exhibited reduced support of early classical pathway-facilitated accumulation of C3. Depending on the antibody isotype, group IV MAbs slightly or markedly enhanced early binding of C3 but had no effect on either the rate of C3 accumulation or the amount of bound C3. When the classical pathway was blocked, group II and III MAbs markedly suppressed C3 binding that normally would have occurred via the alternative pathway. In contrast, MAbs of group IV had no effect on alternative pathway-mediated C3 binding. These results indicate that anticapsular antibodies with different epitope specificities may have distinct regulatory effects on activation and binding of C3.
Figures



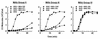

References
-
- Baker C J, Rench M A, Edwards M S, Carpenter R J, Hays B M, Kasper D L. Immunization of pregnant women with a polysaccharide vaccine of group B Streptococcus. N Engl J Med. 1988;319:1180–1220. - PubMed
-
- Bhattacharjee A K, Bennett J E, Glaudemans C P J. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis. 1984;6:619–624. - PubMed
-
- Bjornson A B, Bjornson H S. Participation of immunoglobulin and the alternative complement pathway in opsonization of Bacteroides fragilis and Bacteroides thetaiotaomicron. J Infect Dis. 1978;138:351–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

