Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo
- PMID: 9529102
- PMCID: PMC108109
- DOI: 10.1128/IAI.66.4.1718-1725.1998
Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo
Abstract
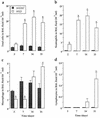
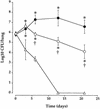
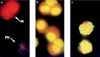
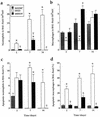
Bordetella pertussis induces in vitro apoptosis of murine alveolar macrophages by a mechanism that is dependent on expression of bacterial adenylate cyclase-hemolysin. Using a murine respiratory model, we found in this study that intranasal infection with a parental B. pertussis strain, but not with an isogenic variant deficient in the expression of all toxins and adhesins, induced a marked neutrophil accumulation in the bronchoalveolar lavage fluid and an early decrease in macrophage numbers. These phenomena paralleled a time-dependent rise in the proportion of apoptotic nuclei, as detected by flow cytometry, and of macrophages which had engulfed apoptotic bodies. Apoptotic death of bronchopulmonary cells was observed exclusively following intranasal infection with bacteria reisolated from lungs of infected animals and not with B. pertussis collected after in vitro subculture. Using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling technique coupled to fluorescence microscopy and morphological analysis, we established that the apoptotic cells in bronchoalveolar lavage fluids were neutrophils and macrophages. Histological analysis of the lung tissues from B. pertussis-infected mice showed increased numbers of apoptotic cells in the alveolar compartments. Cellular accumulation in bronchoalveolar lavage fluids and apoptosis of alveolar macrophages were significantly attenuated in mice infected with a mutant deficient in the expression of adenylate cyclase-hemolysin, indicating a role of this enzyme in these processes.
Figures



References
-
- Ameisen J C, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. - PubMed
-
- Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. - PubMed
-
- Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. - PubMed
-
- Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. - PubMed
-
- Douglas R S, Tarshis A D, Pletcher C H, Nowell P C, Moore J S. A simplified method for the coordinate examination of apoptosis and surface phenotype of murine lymphocytes. J Immunol Methods. 1995;188:219–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

