Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band
- PMID: 9529381
- PMCID: PMC25310
- DOI: 10.1091/mbc.9.4.829
Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band
Abstract
The myofibrils of cross-striated muscle fibers contain in their M bands cytoskeletal proteins whose main function seems to be the stabilization of the three-dimensional arrangement of thick filaments. We identified two immunoglobin domains (Mp2-Mp3) of M-protein as a site binding to the central region of light meromyosin. This binding is regulated in vitro by phosphorylation of a single serine residue (Ser76) in the immediately adjacent amino-terminal domain Mp1. M-protein phosphorylation by cAMP-dependent kinase A inhibits binding to myosin LMM. Transient transfection studies of cultured cells revealed that the myosin-binding site seems involved in the targeting of M-protein to its location in the myofibril. Using the same method, a second myofibril-binding site was uncovered in domains Mp9-Mp13. These results support the view that specific phosphorylation events could be also important for the control of sarcomeric M band formation and remodeling.
Figures

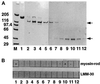

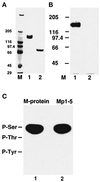
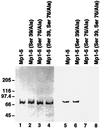

References
-
- Andersson S, Davis DN, Dahlbäck H, Jörnval H, Russel DW. Cloning, structure and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. 3rd ed. New York: Wiley and Sons, Inc.; 1995.
-
- Bähler M, Wallimann T, Eppenberger HM. Myofibrillar M-band proteins represent constituents of native thick filaments, frayed filaments and bare zone assemblages. J Muscle Res Cell Motil. 1985;6:783–800. - PubMed
-
- Carlsson E, Grove BK, Wallimann T, Eppenberger HM, Thornell L-E. Myofibrillar M-band proteins in rat skeletal muscles during development. Histochemistry. 1990;95:27–35. - PubMed
-
- Casnellie JE. Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Methods Enzymol. 1991;200:115–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

