Fluid-phase markers in the basolateral endocytic pathway accumulate in response to the actin assembly-promoting drug Jasplakinolide
- PMID: 9529391
- PMCID: PMC25321
- DOI: 10.1091/mbc.9.4.957
Fluid-phase markers in the basolateral endocytic pathway accumulate in response to the actin assembly-promoting drug Jasplakinolide
Abstract
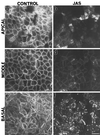
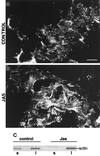
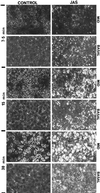
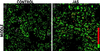
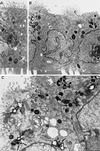
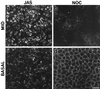
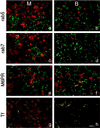
To investigate the role of filamentous actin in the endocytic pathway, we used the cell-permeant drug Jasplakinolide (JAS) to polymerize actin in intact polarized Madin-Darby canine kidney (MDCK) cells. The uptake and accumulation of the fluid-phase markers fluorescein isothiocyanate (FITC)-dextran and horseradish peroxidase (HRP) were followed in JAS-treated or untreated cells with confocal fluorescence microscopy, biochemical assays, and electron microscopy. Pretreatment with JAS increased the uptake and accumulation of fluid-phase markers in MDCK cells. JAS increased endocytosis in a polarized manner, with a marked effect on fluid-phase uptake from the basolateral surface but not from the apical surface of polarized MDCK cells. The early uptake of FITC-dextran and HRP was increased more than twofold in JAS-treated cells. At later times, FITC-dextran and HRP accumulated in clustered endosomes in the basal and middle regions of JAS-treated cells. The large accumulated endosomes were similar to late endosomes but they were not colabeled for other late endosome markers, such as rab7 or mannose-6-phosphate receptor. JAS altered transport in the endocytic pathway at a later stage than the microtubule-dependent step affected by nocodazole. JAS also had a notable effect on cell morphology, inducing membrane bunching at the apical pole of MDCK cells. Although other studies have implicated actin in endocytosis at the apical cell surface, our results provide novel evidence that filamentous actin is also involved in the endocytosis of fluid-phase markers from the basolateral membrane of polarized cells.
Figures






Similar articles
-
Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells.J Cell Biol. 1993 Feb;120(3):695-710. doi: 10.1083/jcb.120.3.695. J Cell Biol. 1993. PMID: 8381123 Free PMC article.
-
Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1.Mol Biol Cell. 2000 Jan;11(1):287-304. doi: 10.1091/mbc.11.1.287. Mol Biol Cell. 2000. PMID: 10637309 Free PMC article.
-
Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters.EMBO J. 1985 Nov;4(11):2781-92. doi: 10.1002/j.1460-2075.1985.tb04004.x. EMBO J. 1985. PMID: 4065093 Free PMC article.
-
Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton.Traffic. 2001 Mar;2(3):149-59. doi: 10.1034/j.1600-0854.2001.020301.x. Traffic. 2001. PMID: 11260520 Review.
-
Jasplakinolide. An actin-specific reagent that promotes actin polymerization.Methods Mol Biol. 2001;161:109-20. doi: 10.1385/1-59259-051-9:109. Methods Mol Biol. 2001. PMID: 11190499 Review. No abstract available.
Cited by
-
Non-covalent assembly of meso-tetra-4-pyridyl porphine with single-stranded DNA to form nano-sized complexes with hydrophobicity-dependent DNA release and anti-tumor activity.Nanomedicine. 2014 Feb;10(2):451-61. doi: 10.1016/j.nano.2013.07.019. Epub 2013 Aug 27. Nanomedicine. 2014. PMID: 23988714 Free PMC article.
-
Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes.J Am Chem Soc. 2008 May 7;130(18):5947-53. doi: 10.1021/ja710644g. Epub 2008 Apr 11. J Am Chem Soc. 2008. PMID: 18402449 Free PMC article.
-
Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP.J Cell Biol. 2000 Feb 7;148(3):519-30. doi: 10.1083/jcb.148.3.519. J Cell Biol. 2000. PMID: 10662777 Free PMC article.
-
Evidence for secretion-like coupling involving pp60src in the activation and maintenance of store-mediated Ca2+ entry in mouse pancreatic acinar cells.Biochem J. 2003 Feb 15;370(Pt 1):255-63. doi: 10.1042/BJ20021505. Biochem J. 2003. PMID: 12423207 Free PMC article.
-
Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein.Mol Biol Cell. 1999 Feb;10(2):501-13. doi: 10.1091/mbc.10.2.501. Mol Biol Cell. 1999. PMID: 9950691 Free PMC article.
References
-
- Bomsel M, Parton R, Kuznetsov SA, Shroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:739–731. - PubMed
-
- Brown D, Sabolic I. Endosomal pathways for water channel and proton pump recycling in kidney epithelial cells. J Cell Sci Suppl. 1993;17:49–59. - PubMed
-
- Brown D, Stow JL. Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev. 1996;76:245–297. - PubMed
-
- Bubb MR, Senderowicz AMJ, Sausville EA, Duncan KLK, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

