Major facilitator superfamily
- PMID: 9529885
- PMCID: PMC98904
- DOI: 10.1128/MMBR.62.1.1-34.1998
Major facilitator superfamily
Abstract
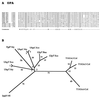
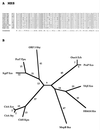
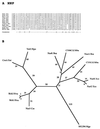
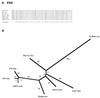
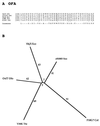
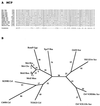
The major facilitator superfamily (MFS) is one of the two largest families of membrane transporters found on Earth. It is present ubiquitously in bacteria, archaea, and eukarya and includes members that can function by solute uniport, solute/cation symport, solute/cation antiport and/or solute/solute antiport with inwardly and/or outwardly directed polarity. All homologous MFS protein sequences in the public databases as of January 1997 were identified on the basis of sequence similarity and shown to be homologous. Phylogenetic analyses revealed the occurrence of 17 distinct families within the MFS, each of which generally transports a single class of compounds. Compounds transported by MFS permeases include simple sugars, oligosaccharides, inositols, drugs, amino acids, nucleosides, organophosphate esters, Krebs cycle metabolites, and a large variety of organic and inorganic anions and cations. Protein members of some MFS families are found exclusively in bacteria or in eukaryotes, but others are found in bacteria, archaea, and eukaryotes. All permeases of the MFS possess either 12 or 14 putative or established transmembrane alpha-helical spanners, and evidence is presented substantiating the proposal that an internal tandem gene duplication event gave rise to a primordial MFS protein prior to divergence of the family members. All 17 families are shown to exhibit the common feature of a well-conserved motif present between transmembrane spanners 2 and 3. The analyses reported serve to characterize one of the largest and most diverse families of transport proteins found in living organisms.
Figures











References
-
- Abe K, Ruan Z S, Maloney P C. Cloning, sequencing, and expression in Escherichia coli of OxlT, the oxalate:formate exchange protein of Oxalobacter formigenes. J Biol Chem. 1996;271:6789–6793. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ambudkar S V, Maloney P C. Characterization of phosphate:hexose 6-phosphate antiport in membrane vesicles of Streptococcus lactis. J Biol Chem. 1984;259:12576–12585. - PubMed
-
- Anantharam V, Allison M J, Maloney P C. Oxalate:formate exchange: the basis for energy coupling in Oxalobacter. J Biol Chem. 1989;264:7244–7250. - PubMed
-
- Bailey T L, Elkan C. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. Menlo Park, Calif: AAAI Press; 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers; pp. 28–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

