Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli
- PMID: 9529891
- PMCID: PMC98910
- DOI: 10.1128/MMBR.62.1.181-203.1998
Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli
Abstract
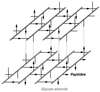
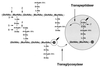
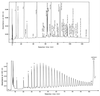
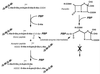
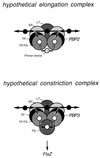
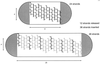
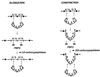
To withstand the high intracellular pressure, the cell wall of most bacteria is stabilized by a unique cross-linked biopolymer called murein or peptidoglycan. It is made of glycan strands [poly-(GlcNAc-MurNAc)], which are linked by short peptides to form a covalently closed net. Completely surrounding the cell, the murein represents a kind of bacterial exoskeleton known as the murein sacculus. Not only does the sacculus endow bacteria with mechanical stability, but in addition it maintains the specific shape of the cell. Enlargement and division of the murein sacculus is a prerequisite for growth of the bacterium. Two groups of enzymes, hydrolases and synthases, have to cooperate to allow the insertion of new subunits into the murein net. The action of these enzymes must be well coordinated to guarantee growth of the stress-bearing sacculus without risking bacteriolysis. Protein-protein interaction studies suggest that this is accomplished by the formation of a multienzyme complex, a murein-synthesizing machinery combining murein hydrolases and synthases. Enlargement of both the multilayered murein of gram-positive and the thin, single-layered murein of gram-negative bacteria seems to follow an inside-to-outside growth strategy. New material is hooked in a relaxed state underneath the stress-bearing sacculus before it becomes inserted upon cleavage of covalent bonds in the layer(s) under tension. A model is presented that postulates that maintenance of bacterial shape is achieved by the enzyme complex copying the preexisting murein sacculus that plays the role of a template.
Figures








References
-
- Asoh S, Matsuzawa H, Ishino F, Strominger J L, Matsuhashi M, Ohta T. Nucleotide sequence of the pbpA gene and characteristics of the deduced amino acid sequence of penicillin-binding protein 2 of Escherichia coli K12. Eur J Biochem. 1986;160:231–238. - PubMed
-
- Bayer M E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968;53:395–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

