Flow-induced arterial remodeling in rat mesenteric vasculature
- PMID: 9530199
- PMCID: PMC11793876
- DOI: 10.1152/ajpheart.1998.274.3.H874
Flow-induced arterial remodeling in rat mesenteric vasculature
Abstract
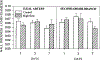
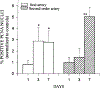
This study was designed to characterize in vivo arterial remodeling of male Wistar rat small mesenteric arteries exposed to varying levels of elevated blood flow in the presence of normal arterial pressure. Through a series of arterial ligations, respective ileal artery and second-order branch blood flows acutely increased approximately 36 and approximately 170% over basal levels. Their respective diameters increased 12 and 38% and their wall area increased 58 and 120% in a time-dependent fashion between 1 and 7 days postlitigation compared with same-animal control vessels. Medical extracellular connective tissue increased concomitantly with medical wall hypertrophy. Immunostaining for proliferating cell nuclear antigen and nuclear profile analyses suggests that both smooth muscle and endothelial cell hyperplasia contribute to flow-induced vascular remodeling. The initial stimulus in this model is flow-mediated shear stress, with possible augmentation by hoop stress, which is increased approximately 7% by the resultant vasodilation. Stable wall thickness-to-lumen diameter ratios at 1, 3, and 7 days, however, suggest chronic hoop stress is tightly regulated and remains constant. The model described herein allows analyses of two arteries with different degrees of flow elevation within the same animal and demonstrates that the magnitude of vessel remodeling in vivo is directly dependent on the duration of flow elevation after abrupt arterial occlusion.
Figures
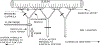




References
-
- Bardy N, Karillon GJ, Merval R, Samuel J-L, and Tedgui A Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ. Res. 77: 684–694, 1995. - PubMed
-
- Baroja A, de la Hoz C, Alvarez A, Ispizua A, Bilboa J, and de Gandarias JM Genesis and evolution of high-ploidy tumour cells evaluated by means of the proliferation markers p34 (cdc2), cyclin B1, PCNA, and [3H]thymidine. Cell Prolif. 29: 89–100, 1996. - PubMed
-
- Cooke JP, Stamler J, Andon N, Davies PF, McKinley G, and Loscalzo J Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am. J. Physiol. 259 (Heart Circ. Physiol. 28): H804–H812, 1990. - PubMed
-
- D’Angelo G, and Meininger GA Transduction mechanisms involved in the regulation of myogenic activity. Hypertension 23: 1096–1105, 1994. - PubMed
-
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, and West MJ Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96: 379–394, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

