A coactivator of pre-mRNA splicing
- PMID: 9531537
- PMCID: PMC316672
- DOI: 10.1101/gad.12.7.996
A coactivator of pre-mRNA splicing
Abstract
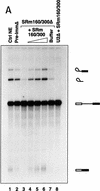
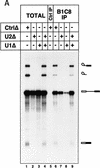
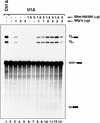
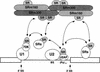
The nuclear matrix antigen recognized by the monoclonal antibody (mAb) B1C8 is a novel serine (S) and arginine (R)-rich protein associated with splicing complexes and is named here SRm160 (SR-related matrix protein of 160 kD). SRm160 contains multiple SR repeats, but unlike proteins of the SR family of splicing factors, lacks an RNA recognition motif. SRm160 and a related protein SRm300 (the 300-kD nuclear matrix antigen recognized by mAb B4A11) form a complex that is required for the splicing of specific pre-mRNAs. The SRm160/300 complex associates with splicing complexes and promotes splicing through interactions with SR family proteins. Binding of SRm160/300 to pre-mRNA is normally also dependent on U1 snRNP and is stabilized by U2 snRNP. Thus, SRm160/300 forms multiple interactions with components bound directly to important sites within pre-mRNA. The results suggest that a complex of the nuclear matrix proteins SRm160 and SRm300 functions as a coactivator of pre-mRNA splicing.
Figures










References
-
- Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. - PubMed
-
- Alzhanova-Ericsson AT, Sun X, Visa N, Kiseleva E, Wurtz T, Daneholt B. A protein of the SR family binds extensively to exonic Balbiani ring pre-mRNA and accompanies the RNA from gene to the nuclear pore. Genes & Dev. 1996;10:2881–2893. - PubMed
-
- Amrein H, Hedley ML, Maniatis T. The role of specific protein-RNA interactions and protein-protein interactions in positive and negative control of pre-mRNA splicing by transformer 2. Cell. 1994;76:735–746. - PubMed
-
- Barabino SML, Blencowe BJ, Ryder U, Sproat BS, Lamond AI. Targeted snRNP depletion reveals an additional role for mammalian U1 snRNP in spliceosome assembly. Cell. 1990;63:293–302. - PubMed
-
- Berget SM. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
